Conclusions
Compared to nearby cold springs and Diamond Lake to the north, Crater Lake has anomalously high concentrations of dissolved Na, Li, Cl, S04, and B. Additionally, the 6D and 6180 values for the lake water are significantly higher (heavier) than for cold-spring waters. The isotopic difference between lake water and cold-spring water is caused by evaporation. The water isotopes, SD and 6180, determine an evaporation line between Annie Spring and Crater Lake water having a slope of five, which is typical for evaporated waters. Diamond Lake also plots along this evaporation line. The chemical enrichments in Crater Lake, however, cannot be explained by evaporation.
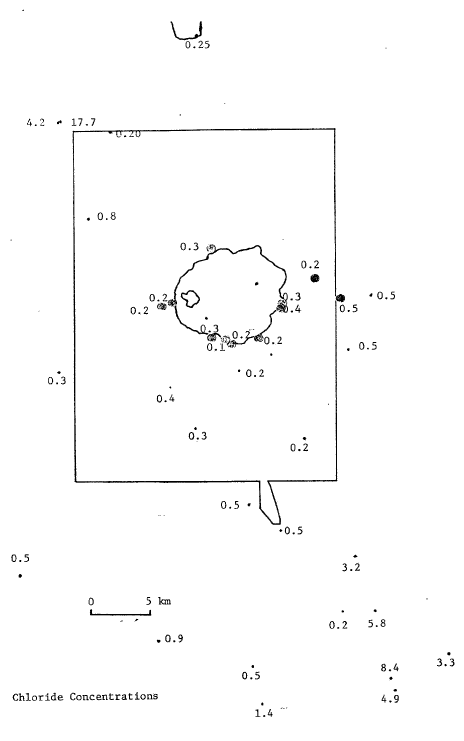
No one spring was identified as being the outlet of Crater Lake. We see no evidence for any spring containing more than ten percent lake water. Crater Spring in the NW part of the park (see figure 1 and 2) may contain some Crater Lake water. The evidence for this is that Crater Spring plots along a mixing line between Crater Lake water and the meteoric water line and also that it contains some Cl and plots along a 6D – Cl mixing line between Crater Lake and dilute spring waters. If Crater Spring does contain Crater Lake water, it cannot contain more than seven percent lake water. Oasis Spring, in the same vicinity, may also contain some Crater Lake water.
Crater Lake appears to be well mixed based on chemical and isotopic analyses. The concentrations of SiO2, Cl, Na, Li, SO4, and B do not vary significantly as a function of depth. The 6D and 6180 values are remarkably uniform throughout the lake water. Tritium data indicate that recent precipitation rapidly mixes with many volumes of lake water in the near surface. The heat flow values reported by Williams and Von Herzen (1983) are sufficient to cause small density gradients that allow the lake to convect in the deeper levels. This Rayleigh convection is apparently able to mix the lake water thoroughly over a 1-year period because there are no major ion chemical gradients found in Crater Lake (Simpson, 1970).
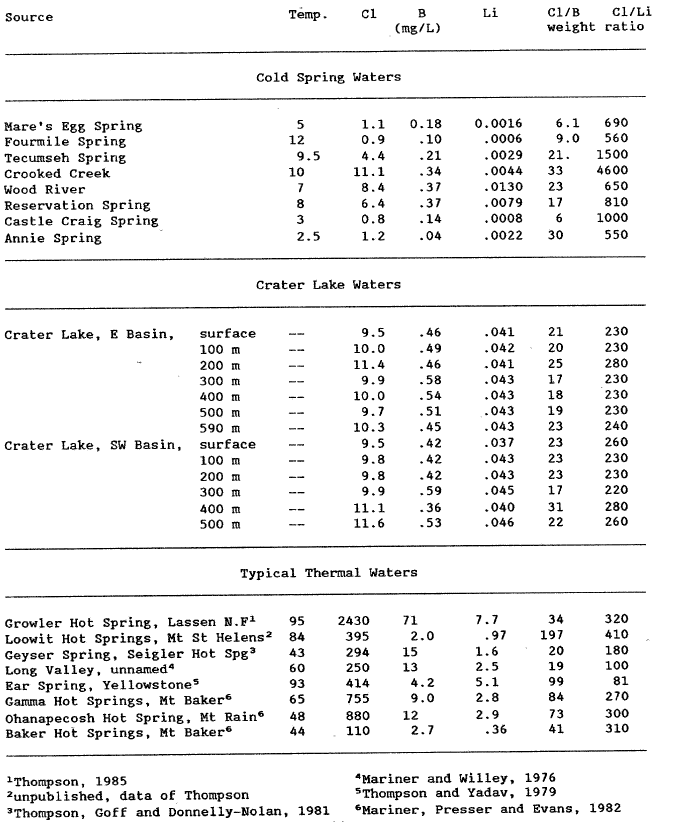
Thermal water generally contains moderate to high concentrations of dissolved boron, chloride, and lithium. Crater Lake also appears to have an anomalously high Li concentration compared to other waters in this area. As is observed from table la and table 4, other cold springs can have somewhat elevated chloride concentrations and similar Cl/B weight ratios thus negating their overall usefulness. The Cl/Li weight ratio may be useful in assessing if the Cl is also derived from a thermal water. The mean Cl/Li weight ratio for Crater Lake is calculated to be 242, which is comparable to thermal waters from volcanic environments, 81 – 410, and is substanially lower than the lowest cold-spring ratio (550) at Annie Spring. This supports the hypothesis that Crater Lake contains thermal water and also explains the elevated Ma, Li, Cl, S04, and B concentrations. With the present data it is not possible to assess a) the amount of, b) the temperature of, or c) the composition of this inferred thermal water.
 |
|
Table 4. Analyses of B and Li in partially evaporated samples of lake water collected in 1985, recalculated to original concentrations, and values for other western U. S. Hot Springs |
 |
|
Figure 2. Chloride concentrations (mg/L) in spring waters from the Crater Lake area. |