Chemical Analyses of Waters from Crater Lake, Oregon, and Nearby Springs
by J. Michael Thompson, L. Douglas White and Manuel Nathenson
Chloride Analyses of Crater Lake Water
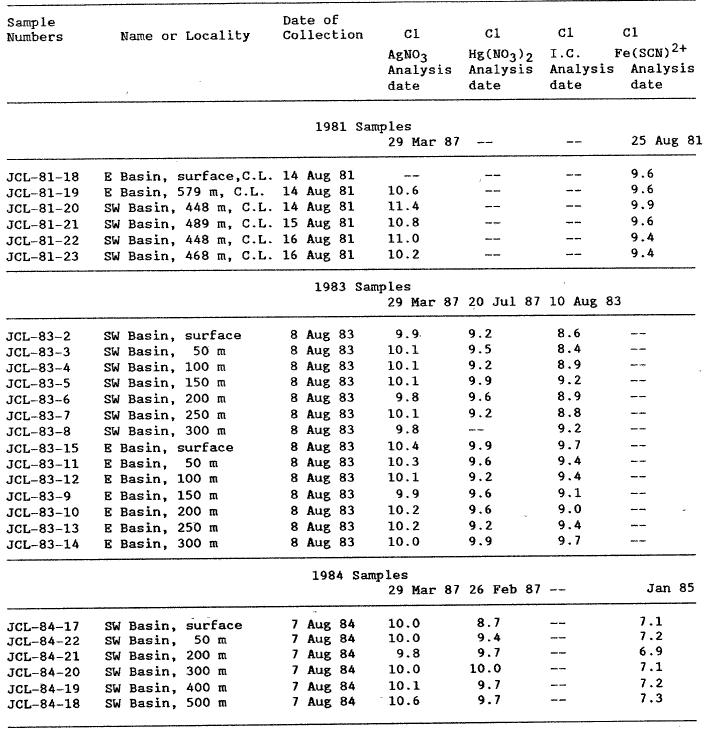
Because of inconsistencies in chloride values from two methods that became apparent when analyzing the last samples collected, we reanalyzed our Crater Lake water samples by at least two, and generally three, different methods for dissolved C1 (table 2). From the data in table 2, different analytical methods yield different Cl concentrations. Some measured C1 concentrations differ more than 1 mg/L.
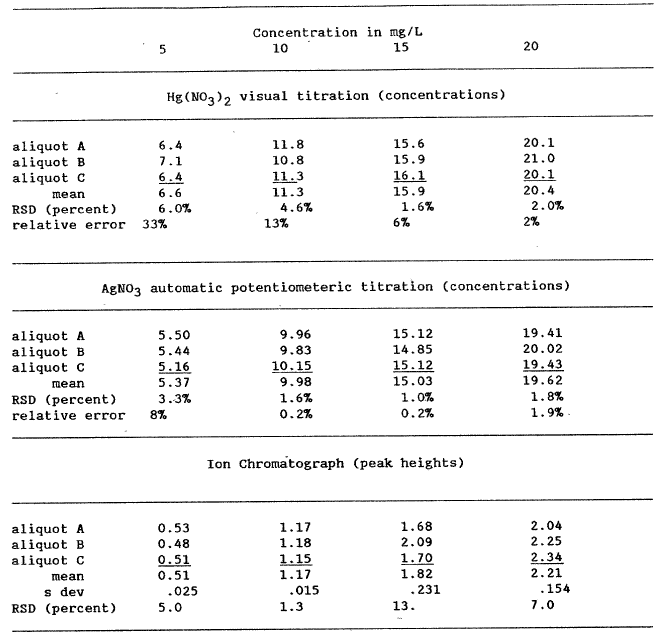
These determinations pointed out the requirement for more information on both the precision and accuracy of the chloride procedures followed. To address this analytical problem, we prepared two different experiments. The first was to reanalyze the 1981, 1983, and 1984 lake water samples using analytical techniques not initially employed. The second was to prepare four solutions containing 5, 10, 15, and 20 mg/L Cl and then to analyze each of the four solutions three times by each analytical method employed: ion chromatography, an automated AgN03 titration, and a manual Hg(N03)2 titration. The results of these analyses are reported in table 3.
To standardize the titrants for the two titration procedures, we used the method described by Fishman and Friedman (1985). After standardization of the titrant, the various standards were titrated 3 times and each individual concentration calculated (table 3). The mean and standard deviation are also reported in table 3. Because preparing standards for the ion chromatographic procedure is essentially a repeat of preparing the four standard Cl solutions, the error for this method was the variations in the peak heights. As can be observed from the RSDs calculated in table 3, some values are quite precise but others have significant variations.
The regression that passes nearest the origin using mean values is the ion chromatographic line, intercept a = -.014. Using the total data set, the best regression line is that for the automated AgNO3 titration, a = .545. Using the mean values, the Hg(N03 )2 titration has good overall precision, but the worst intercept, a = 2.08. The method of choice seems to be the automated AgNO3 titration because it is easy, rapid, and accurate. The Cl concentrations reported in table lb for Crater Lake water samples collected since 1982 were determined using the automated AgNO3 titration. The 1981 samples apparently evaporated too much for an adequate comparison.
Fishman and Pyen (1979) reported results of ion chromatographic (IC) and automated colorimetric (AC) Cl analyses for numerous surface waters. Assuming that the AC method is correct, the mean difference between the IC and AC is -0.76 (std. dev. = 1.30) for samples containing less than 20 mg/L C1. A similar comparison can be made for our 1983 Crater Lake data in table 2. Assuming the AgNO3 procedure is correct, the mean difference between methods is 0.95 (std dev = .34). This suggests a constant error of about 1 mg/L between the IC method and any other method.
 |
|
Table 2. Chloride Analyses of Crater Lake Waters (Cone. in mg/L) |
 |
|
Table 3. Comparison of mercuric nitrate and silver nitrate titrations and ion chromatography determinations for chloride Concentration in mg/L |
***previous*** — ***next***

