Hydrologic Benchmark Network Stations in the Western U.S. 1963-95 (USGS Circular 1173-D)
Historical Water-Quality Data and Time-Series Trends
The data set for the Crater Lake HBN station analyzed for this report includes 91 water quality samples that were collected from June 1967 through September 1995. Sampling frequency averaged only three samples per year because of closure of the access road in winter. Samples from the early part of the period of record probably were analyzed at a USGS district laboratory in Portland, Oreg. (Durum, 1978). After establishment of the central laboratory system, samples were analyzed at the Salt Lake City, Utah, laboratory from 1973 to 1975 and the NWQL in Arvada, Colo., from 1976 to 1995. Lake stage for Crater Lake (station 11492200) is available beginning in October 1961, and daily water temperature at the station has been measured since October 1963.
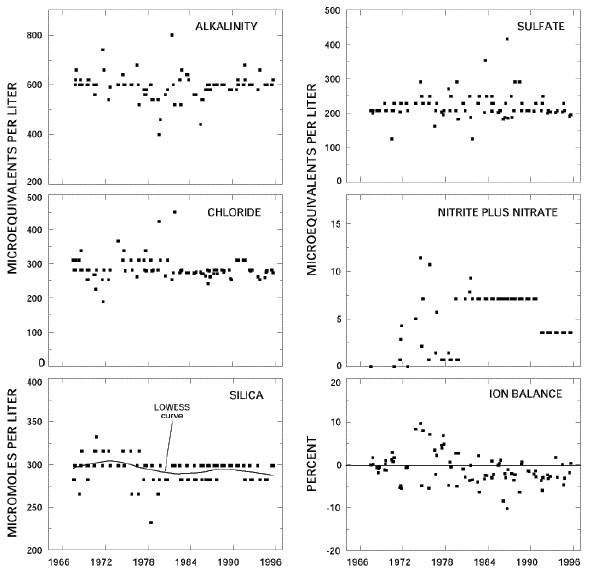
Calculated ion balances for 86 samples that have complete major-ion analyses are shown in figures 19a and 19b. Graphs showing temporal variation of discharge, field pH, majorion concentrations, and ion balance in Crater Lake, Oregon. Ion balances ranged from – 10 to +9.6 percent, and 85 percent of the samples had values within the ±5-percent range, indicating that the analytical results are of high quality. The average ion balance for all samples was -1.0 percent, and 65 percent of samples had a slight excess of measured cations compared to measured anions, indicating that unmeasured constituents, such as organic anions, may have contributed a small amount to the ionic content of lake water at this HBN station. Time-series plots of the major dissolved constituents were inspected for evidence of method-related effects (fig. 19). The most notable pattern is in field pH, which increased rather abruptly in 1988. Several uncharacteristically high calcium and sodium concentrations were measured during the 1970’s. In addition, the scatter in calcium, alkalinity, and chloride concentrations decreased noticeably during the period of record. Because the surface-water chemistry at this station should be relatively stable due to the long residence time of water in the lake, these patterns probably are caused by sampling or analytical artifacts rather than by natural variability in lake chemistry.
Figure 19a. Graphs showing temporal variation of discharge, field pH, major-ion concentrations, and ion balance in Crater Lake, Oregon
Figure 19b. Graphs showing temporal variation of discharge, field pH, major-ion concentrations, and ion balance in Crater Lake, Oregon – Continued
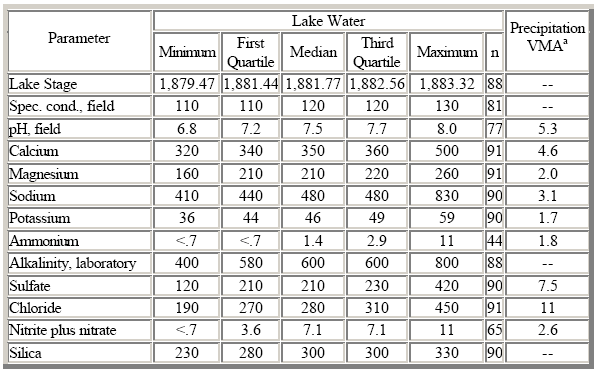
The median concentrations and ranges of major dissolved constituents in lake water collected at the HBN station and VWM concentrations in bulk precipitation collected in Crater Lake National Park are presented in table 34, and correlations between lake stage and the major solutes are presented in table 35. Precipitation chemistry in the park is very dilute and slightly acidic and has a VWM pH of 5.3. The predominant cations in precipitation were hydrogen ion, calcium, and sodium, which contributed 27, 25, and 17 percent of the total cation charge, respectively. The predominant anions were sulfate and chloride, which accounted for 36 and 51 percent of the total anions, respectively. Lakewater samples from Crater Lake are moderately concentrated and well buffered; specific conductance ranged from 110 to 130 mS/cm, and alkalinity ranged from 400 to 800 meq/L (table 34). The predominant solutes in lake water were sodium, calcium, bicarbonate, silica, and concentrations of most solutes in lake water were much higher than concentrations in precipitation despite the fact that the lake receives 85 percent of its inflow from direct precipitation (Nelson and others, 1993). Sources of solutes other than atmospheric deposition include dissolution of geologic materials on the lakebed, springs and small streams that emanate from the caldera walls, and hydrothermal sources under the lake. The predominance of sodium compared to calcium and the high concentrations of dissolved silica in lake water are consistent with the weathering stoichiometry of sodium-rich volcanic glass and plagioclase minerals in the volcanic bedrock (Nathenson and Thompson, 1990).
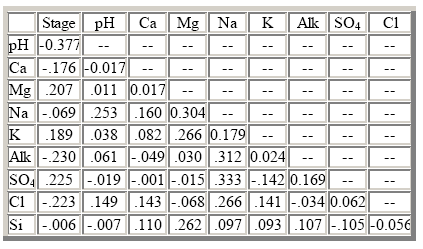
The elevated chloride and sulfate concentrations in lake water cannot be accounted for by either atmospheric deposition or bedrock weathering and may be derived from thermal springs that discharge from the floor of the lake (Van Denburgh, 1968). Biological activity also functions as a control on the solute budget of the lake. For example, diatom activity in the lake removes as much as 30 percent of the dissolved silica introduced from weathering and thermal sources (Nelson and others, 1996). More than 90 percent of the nitrate and ammonium that enters the system in atmospheric deposition is assimilated by algae and subsequently buried in sediments on the bottom of the lake (Dymond and others, 1990). All solute concentrations were poorly correlated with lake stage and with each other because of the extremely constant chemical composition of the lake (table 35). More detailed information on the processes controlling the chemistry of Crater Lake is presented in Nathenson and Thompson (1990), Larson and others (1996), and Nelson and others (1996).
| Table 36. Results of the seasonal Kendall test for trends in lake stage and unadjusted pH and major dissolved constituents, Crater Lake, Oregon, June 1967 through September 1995
[Trends in units of microequivalents per liter per year, except for stage in meters per year, pH in standard units per year, and silica in micromoles per liter per year; <, less than; –, not calculated] a Trend calculated for 1971-95 using a test for censored data. |
The results of the seasonal Kendall test for trends in lake stage and unadjusted dissolved constituents are listed in table 36. Trends were not calculated for the stage-adjusted concentrations because correlations between solute concentrations and lake stage were not significant at the 0.10 probability level. Statistically significant trends were detected in lake stage and unadjusted magnesium, potassium, and silica concentrations at the 0.01 probability level. The LOWESS curve for lake stage in figure 19 shows that the trend in lake stage was primarily caused by declining lake levels at the end of the period of record. Given that climate variability can account for lake-level changes during the last century (Redmond, 1990), this decline was probably caused by a period of dry and warm weather in the south-central part of Oregon that persisted from about 1985 to 1995 (Oregon Climate Service, at URL http://www.ocs.orst.edu, accessed 1998). Trends in water- quality constituents at this HBN station were not expected because the water column is well mixed and the residence time of water in the lake is around 225 years (Collier and others, 1990). In addition, the trends are inconsistent with the results of Larson and others (1996) who reported no long-term changes in lake chemistry during a 10-year limnological study of the lake nor with a comparison of recent and historical data. Closer inspection of the trend results in table 36 reveals that the change in magnesium and silica concentrations was less than 0.1 percent during the entire period of record. Because the analytical precision of these analyses is no better than 5 percent (Fishman and Friedman, 1989), the statistical test probably did not detect measurable changes in the concentrations of these two constituents. The trend in potassium was considerably larger in magnitude than the trends in magnesium and silica and amounted to a total decrease in concentration of about 17 percent, most of which occurred in the latter part of the period of record (fig. 19). The cause of the downward trend in potassium could not be identified. One possibility is a method-related artifact, although there were no documented changes in the analytical technique for potassium since 1965 (Fishman and others, 1994). Alternatively, potassium concentrations may have been affected by the decline in lake level that occurred from 1985 to 1995.
***previous*** — ***next***