Hydrology of Crater, East and Davis Lakes, Oregon by Kenneth N. Phillips
CHEMISTRY OF THE LAKES
By A. S. VAN DENBURGH
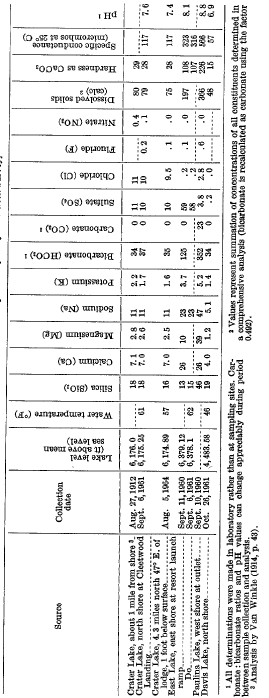
Crater, East, and Davis Lakes all contain only small amounts of dissolved solids (table 13). Nonetheless, each lake is distinctively different chemically because the dilute lake waters are sensitive to the influences of even slightly differing hydrochemical environments. Thus, the character of Davis Lake is governed primarily by the constituents that are most readily drawn out of the soil and underlying rocks by very dilute natural runoff and percolation, a process that is aided by the dissolution of carbon dioxide. The resulting lake water contains only about 50 ppm (parts per million) of dissolved solids, which includes silica, bicarbonate, and very little else. Crater Lake water, with a dissolved-solids concentration of about 80 ppm, is in many respects similar chemically, but in addition it contains noticeable amounts of several hydrochemical products of its volcanic-caldera environment. Only small quantities of the telltale constituents – sodium, sulfate, and chloride-are necessary to draw attention to that environment. At East Lake, where the dissolved constituents of thermal-spring flow are the strongest and overshadowing control, the chemical character of “Davis Lake-type” water is masked almost completely. The result is a calcium sodium bicarbonate sulfate water with four times the dissolved-solids concentration of Davis Lake.
| TABLE 13.-Chemical and physical character of the lakes |
 |
***previous*** — ***next***

