Hydrology of Crater, East and Davis Lakes, Oregon by Kenneth N. Phillips
CHEMISTRY OF THE LAKES
By A. S. VAN DENBURGH
EAST LAKE
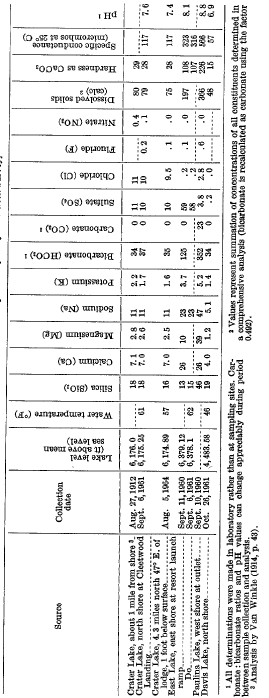
Although the geologic environments of East and Crater Lakes are quite similar-each occupies a volcanic caldera of Holocene age-their chemical characters are dissimilar. East Lake contains about 200 ppm of dissolved solids, more than twice the amount in Crater Lake. The principal cations are calcium, sodium, and magnesium, which are present in almost equal amounts, based on equivalents per million. Bicarbonate and sulfate are by far the most abundant anions, whereas the concentration of chloride is remarkably small (about 0.2 ppm; table 13).
Perhaps the most important source of dissolved solids in the East Lake basin is the discharge of hot springs situated in one known shallow area of the lake basin below the present water level; similar springs may occur in deeper parts of the lake as well. The analysis of a lakewater sample collected in the area of spring activity at the southeastern shore shows that the major constituents contributed by the springs probably rare silica, bicarbonate, and sulfate; the identity of the principal cation (s) is uncertain. The springs also emit gaseous hydrogen sulfide, and almost certainly carbon dioxide as well.
Additional less important sources of dissolved solids are: Hydrochemical alteration and decomposition of the lakebed below the present lake level, percolation and surface runoff into the 1.5-square-mile lake from the surrounding 6.0-square-mile drainage basin, and precipitation falling directly into the lake. Hydrochemical action of the lake water may be accelerated in areas of hot-spring activity by an abundance of carbon dioxide. The carbon dioxide tends to inhibit the increase in pH normally associated with the decomposition or alteration of some common minerals, such as alkaline-earth carbonates, feldspars, micas, and certain clay minerals. As the pH of a solution that surrounds the altering mineral increases in the range from pH 7 to 10, the tendency to be altered (or, in the case of minerals such as calcite, to be dissolved) decreases. Thus, the presence of gaseous carbon dioxide aids the decomposition processes because dissolution of the gas and subsequent dissociation of the carbonic acid initially formed provides hydrogen ions that depress the pH.
The relatively dilute character of the lake water, in spite of the dissolved-solids contribution from hot springs, supports the hydrologic evidence (p. E29) that some of the water and its dissolved material are leaving the lake rather than accumulating. Because there is no indication that the lake has ever overflowed into adjacent Paulina Lake, subsurface leakage is the only means of water loss with concurrent dissolved-solids removal. If, as suggested on page E29, leakage from the lake is about 2.3 cfs, the annual loss of dissolved solids would be about 450 tons, or 3 percent of the lake’s estimated 15,000-ton dissolved-solids load. The magnitude of annual dissolved-solids depletion in East Lake is by itself ample evidence that lakes in leaky basins remain dilute, even if the leakage is slight.
A logical path for the leakage would be westward from East Lake (alt about 6,380 ft) to adjacent Paulina Lake (alt about 6,330 ft). However, the almost ninefold difference in magnitude between the outflow from Paulina Lake (about 20 cfs) and the estimated leakage from East Lake (about 2.3 cfs) prevents the detection of leakage to Paulina Lake from chemical criteria alone, despite the marked differences in chemical character (table 13).
| TABLE 13.-Chemical and physical character of the lakes |
 |
***previous*** — ***next***

