Report of the Sec. of the Interior under Sec. 7 of Public Law 100-443 on the Presence or Absence of Significant Thermal Features Within Crater Lake National Park, 1992
V. Appendices
B. Peer Review Report
Crater Lake
March 1991
Peer Review of Research Program
and Draft Report on Studies of
Hydrothermal Processes in Crater Lake, Oregon
A Report of Field Studies Conducted in 1989 for the National Park Service
PANEL MEMBERS
Dr. Charles R. Goldman, Chairman
Dr. David D. Blackwell
Dr. Wilfred A. Elders
Dr. Joris M. Gieskes
Dr. James McClain
Dr. Kenneth H. Nealson
Dr. H. James Simpson
CORRESPONDING MEMBERS
Dr. Jorg Imberger
Dr. Alan D. Jassby
TABLE OF CONTENTS
Pace
Executive Summary
I. Introduction: The Review Process
II. Crater Lake Overview
III. Limnology
A. Hydrothermal inputs and water quality
B. The extent of water quality degradation
C. Nutrients and decrease in water clarity
IV. Microbiology of Benthic Mat Communities
A. Introduction and general comments
B. Report
C. Pure culture studies
D. Enzyme assays
E. Dissolved substrate analyses
F. Other relevant data
G. Analyses, interpretations, and conclusions
H. Additional studies and suggestions
V. Geophysics and Geology
A. Introduction and general comments
B. Report
C. The discovery of spires near Skell Head
D. Comments on mixing calculations
E. Comments on “box” modeling effort
VI. Geochemistry
A. Introduction
B. Inorganic constituents
C. Pore water chemistry
D. Oxygen mass balance
E. Rare Earth elements
F. Geochemical modeling
G. Geothermometry
H. Geochemistry of sediments
I. Investigations with isotopes and freons
J. Additional studies and suggestions
VII. Summary
A. Important results from 1989 and 1990 field seasons.
B. Other comments
VIII. References
Executive Summary
The Panel has discussed in great detail exactly what warm, slightlysaline inflows to Crater Lake should be called, and settled on “Salinity- and Heat-Enriched Fluids”, or SHEF. We considered using “hydrothermal water”, the American Geological Institute’s definition of which is “subsurface water whose temperature is high enough to make it geologically or hydrologically significant, whether or not it is hotter than the rock containing it”, and decided to avoid the strict implication of this definition. The Panel also considered using either SELTHF (“salinity-enriched low temperature hydrothermal fluids”) or SAM (“slightly-warmer and more-saline water”), and finally settled on SHEF as a good and understandable compromise.
Some concern has been expressed in the limnology section of the Panel’s report on the validity of the original assumption that the lake has actually been losing transparency. A close examination of the available Secchi data suggests that there may not have been a significant loss. Evidence from sedimentary profiles suggests that phytoplankton productivity is correlated with hydrothermal fluid inputs on a 100-1000 year time scale. But in shorter time scales, anthropogenic influences may be much greater than hydrothermal ones. Refinement of the nitrogen budget, as noted by the previous Panel, remains one of the most important research needs for understanding Crater Lake’s water quality.
Geochemical information presented by Dymond and Collier has shown that an input of SHEF occurs into the deep waters of the lake, particularly in the South Basin. These fluids are primarily sodium/magnesium/calcium bicarbonate waters with a moderate amount of sulfate and chloride, with total dissolved solids (TDS) of about 5 to 7 times higher concentration than the 90 mg/liter TDS of the lake water, which is a very dilute, near-neutral NaCl-S04, water. These SHEF waters are also characterized by enhanced levels of 3He, 222Rn, and reduced iron, but their TDS contents are orders of magnitude lower than those typical for geothermal fluids from a wide variety of environments. Because of their dilute nature and unusual chemistry, caution is necessary in using geochemical geothermometers on these waters, although some evidence suggests that the original temperatures may have been above 19 0C, the highest measured SHEF water temperature. We have no knowledge of the subterranean fluid pathway and, therefore, can only speculate on the depth of origin of the fluids and the temperature of the interaction. Of importance, however, is the observation that SHEF fluids are a major contributor to the salt balance of this lake, with the heat balance being affected only locally. The geochemical evidence agrees with the postulate that the chemistry of the SHEF fluids is affected by water-rock interaction at some elevated, although unspecified, temperatures.
The freon data reported provide the most sensitive indicators currently available of the time scale of deep water ventilation and establish the deep water renewal time to be about two years, assuming a steady-state vertical mixing process. This finding is a critical new result which helps constrain the magnitude of chemical fluxes from SHEF fluids into the deep waters of the lake, averaged over the mean vertical mixing time.
The 222Rn activities observed in samples of deep water clearly establish the depth and general location at which the SHEF fluids are delivered to the deep waters of the lake. The distribution of this tracer in the lake water provides unequivocal evidence of influx of high 222Rn fluids to the deep waters of the lake at the time of sampling during August 1989.
With regard to the microbiological component, the Panel feels that the discovery of the microbial mat communities in Crater Lake was a major finding and may, if followed up, lead to an appreciation of several aspects of biogeochemical cycling in Crater Lake, as well as making possible its comparison with other lacustrine and oceanic environments. Considerable laboratory and fieldwork will be required before the most positive aspects of the microbiology can be realized. Future work should be done in close collaboration with microbiologists and biogeochemists familiar with the biology and biochemistry of iron cycling.
In retrospect, one problem with the report was that its experimental design was limited to consideration of only part of the important relevant issues. Answering the overriding question, “Are there hydrothermal inputs to the lake which are significant in influencing its transparency or its ecology?”, breaks down into answering the following more specific questions: (a) “What is the nature and size of detectable or probable inputs of hotter water into the lake?”; (b) “Is the chemical budget from such hotter sources significant in affecting the lake’s clarity or ecology?”; and (c) “Is the thermal budget from such sources significant in affecting the clarity and ecology?”. At the outset, it seems that the investigators made the implicit assumption that finding hotter water inputs of any level of intensity would be enough to show that they were significant, i.e., they are defined as hydrothermal. Thus, attention was focused almost entirely on question (a), above, so that questions (b) and (c) to some extent still remain unanswered. The research to date has laid much of the groundwork for a better understanding of Crater Lake, and the Panel has been convinced of the input of SHEF to the lake bottom and its significance for lake chemistry. It remains for future studies to determine just how important these inputs are to the dynamics of biological and other processes related to water clarity in Crater Lake. A great effort was made by the principal investigators despite the limited funding available for a research effort which included expensive submersible time.
I. Introduction: The Review Process
In 1989, a scientific controversy regarding possible hydrothermal heating and the roles of hydrothermal fluids in the ecology and particularly the clarity of Crater Lake, Oregon, resulted in the formation of a special Peer Review Panel to evaluate the research findings and recommend any necessary further study. The main question — whether or not Crater Lake contained significant geothermal features — could not be resolved at the time. The National Park Service sponsored additional research still centered on this issue, and subsequently re-assembled an interdisciplinary Peer Review Panel to evaluate the 1990 draft report summarizing the research on possible geothermal features at Crater Lake.
The 1991 Peer Review Panel consisted of seven regular members and two corresponding members (see Table 1). To the original Panel of Drs. Blackwell, Gieskes, Goldman, McClain and Nealson were added Drs. Elders and Simpson. Drs. Imberger and Jassby were the two corresponding members. The Panel examined relevant documents and met for a technical workshop to consider to what extent geothermal venting and/or a diffuse heat flow were occurring in Crater Lake. Included in the Charge to the Panel was a request to evaluate the adequacy of the research and make recommendations as to the strength of the evidence for both scientists and decision-makers on the geothermal heating question.
After its appointment, the Peer Review Panel received the 1990 draft report and other documents, together with reviews from both the U.S. Bureau of Land Management and the CE Exploration Company. Each Panel member benefited from the additional research information provided at the presentations made by Drs. Dymond and Collier. The Panel also received supplementary information on the modeling effort and reports from its two corresponding members. The Panel considers the recommendation for additional research which has been included at the end of the report to be a vital component.
The Panel met in an open meeting on 14 January 1991 in Corvallis, Oregon at the request of Dr. Gary Larson of the National Park Service. After introduction of the Panel, an attorney for CE Exploration, Mr. Ivan Lewis Gold, made an opening presentation to the Panel regarding their concerns about the report. A summary of the research program and its findings was made by Drs. Collier and Dymond and their associates. Following the presentations, the Panel asked a number of questions, then went into Executive Session on 15 January to discuss the material presented and start work on the Panel’s report. Each Panel member provided an individual report to the Chairman that included suggestions for modification of existing research as well as recommendations for any additional research they felt would contribute to an understanding of Crater Lake. A draft report was first prepared by the Chairman from the individual reports, circulated to the Panel members, then revised for a second review by the Panel before final submittal to the National Park Service.
Table 1
Crater Lake Hydrothermal Peer Review Panel – 1991
Dr. Charles R. Goldman
Chairman, Peer Review Panel
Chairman and Professor of Limnology
Division of Environmental Studies
University of California
Davis, CA 95616
| Dr. David D. Blackwell Hamilton Professor of Geophysics Department of Geological Sciences Southern Methodist University Dallas, TX 75275 |
Dr. Wilfred A. Elders Department of Earth Sciences 438 Geology Building University of California Riverside, CA 92521 |
| Dr. Joris M. Gieskes Scripps Institution of Oceanography University of California La Jolla, CA 92093 |
Dr. Jorg Imberger Centre for Water Research Department of Civil Engineering University of Western Australia Nedlands, W.A. 9009, Australia (Corresponding Member) |
| Dr. Alan D. Jassby Division of Environmental Studies University of California Davis, CA 95616 (Corresponding Member) |
Dr. James McClain Department of Geology University of California Davis, CA 95616 |
| Dr. Ken H. Nealson Centre for Great Lakes Research University of Wisconsin 600 East Greenfield Ave. Milwaukee, WI 53204 |
Dr. H. James Simpson Department of Geological Sciences Lamont-Doherty Geological Observatory of Columbia University Palisades, NY 10964 |
The Panel wishes to emphasize that this report responds only to the 1990 draft report of Collier and Dymond, which was supplemented by their oral presentation. Subsequent changes to their report have not been reviewed by the Panel.
II. Crater Lake Overview
The major evidence regarding the presence or absence of hydrothermal systems in Crater Lake is thermal and geochemical. Geological arguments have not as yet played a major role in the controversy. The pertinent issues include regional setting, bottom bathymetry and other bottom observations, bottom samples, heat transfer, lake thermal mixing dynamics, geochemistry, and microbiology. Each of these will be discussed here. We have presumed from the beginning of our first review (Goldman et al. 1989a) that the research program was designed to test the hypothesis that deep circulating hydrothermal waters are entering the bottom of Crater Lake. It now appears that all members of our Panel are in substantial agreement that some form of fluid input is in fact entering the lake beneath the South and East basins, and this water is responsible for temperatures slightly elevated above the normal ambient lake values along the lake bottom, for most of the chemistry in the lake water, and for the growth of bacterial mats on the lake bottom. Although this report focuses on the work of Dymond and Collier as the authors of the Draft Report (October 26, 1990) which served as the focus for our review, it also utilizes a variety of other sources of information and the extensive collective experience of the Panel.
III. Limnology
Crater Lake is a classic example of a collapsed volcano peak forming a caldera lake. It is the second deepest lake in the Western Hemisphere and is renowned for its clarity and beauty. Extinction coefficients were measured by Utterback et al. in 1942 and color by Smith et al. in 1973. A variety of limnological studies have been in progress for the last decade. These have recently been included in a volume edited by Drake et al. (1990), and summarized in a paper by Goldman (1990) appearing in the same volume.
One of the serious gaps which still remains in the existing limnological data for Crater Lake is knowledge of the lake’s annual mixing regime. Crater Lake has a volume of about 16 cubic kilometers and, as a result, a great capacity to dilute any hydrothermal or cold-groundwater inflows to the deep waters of the lake. Consequently, the importance of knowing the extent of mixing of Crater Lake in any given year is necessary in order to evaluate vertical profiles of both temperature and the lake chemistry as influenced by deep water influxes. Many deep lakes undergo only partial mixing during a winter period that is warmer than usual, or lacks a sufficiently-violent storm to complete vertical mixing during the coldest period when the density difference between surface and deep water is lowest.
As noted in our 1989 report, relative depth (Zr), the maximum depth as a percentage of the mean surface diameter, is a convenient scalar quantity which summarizes the effect of basin morphometry on the likelihood of complete mixing. Herdendorf (1982) tabulated the data necessary to calculate Zr for 164 of the 253 largest natural lakes in the world. Lake Tahoe, for example, has the highest Zr value (1.8) of those lakes and, in comparison to other large lakes, is most prone to incomplete mixing by virtue of its shape (Goldman and Jassby 1990). Crater Lake’s smaller surface area and greater depth give it an even higher Zr value (6.6) than Tahoe, indicating that interannual variability in mixing is also probably quite common.
Determining the extent of vertical mixing requires intensive limnological sampling during the coldest period of the year or immediately following ice-out. Lakes that freeze are usually considered to undergo complete mixing soon after ice-out. In reality, in basins like Crater Lake, thermal stratification may set up without complete mixing even if an ice cover has not been present. From a technical standpoint, determining the depth of mixing during the period of minimum thermal stability is not easy. Neither temperature nor conductivity gradients may be adequate to determine the depth of mixing with precision. In Lake Tahoe, the use of a nitrate profile which develops during the spring and summer from the depletion of near-surface nitrate by phytoplankton proved to be the most sensitive means of determining the depth of mixing (Paerl et al. 1975; Goldman and Jassby 1990). Since nitrate depletion is evident in the surface waters of Crater Lake, this same procedure of doing careful nitrate profiles should be sufficient to improve estimates of the extent of vertical mixing.
A. Hydrothermal inputs and water quality
Interest in hydrothermal inputs stems in part from their potential effects on water quality. It is clear that hydrothermal venting could affect phytoplankton biomass and, hence, water clarity, but the magnitude and nature of this influence is still unknown. Several mechanisms can be suggested a priori:
1. Decreased vertical transport of regenerated nutrients from deep waters due to density stabilization of the water column by hydrothermal fluids with higher salinity than ambient lake water.
2. Increased vertical transport of regenerated nutrients from deep waters due to thermal buoyancy derived from hydrothermal waters added to occasional deep mixing.
3. An increased lake nutrient pool due to loading via hydrothermal influxes, made available in the euphotic zone by wind-driven deep mixing events.
4. Influx of trace elements such as copper, boron, arsenic or other heavy metals, which can be toxic at significant concentrations. Dymond and Collier (1990) offer some tentative evidence based on sediment profiles that primary productivity and hydrothermal activity are positively correlated on a time-scale of 102 to 103 years. If this were true, then mechanisms 1 and 4 would be unlikely and we would have to conclude that hydrothermal fluids probably decreased water clarity by contributing to increased phytoplankton biomass. As Dymond and Collier point out, however, the evidence for this correlation is weak. Further, the sediment profiles are unable to resolve variability at scales of 1 to 10 years, and different mechanisms could be operating at these shorter time scales.
Of mechanisms 2 and 3, Dymond and Collier (1990) favor the latter, arguing that the former could not have sustained the long-term enhanced losses of nutrients to the sediments. In actual fact, long-term enhanced loss of Si may have been sustainable if Si was not limiting phytoplankton biomass. The critical issue is whether long-term loss of the nutrient limiting maximum biomass was significantly enhanced, i.e., whether the increased loss represented a significant fraction of the water column pool for this nutrient. This question still appears to be unanswered. Although the “Salinity- and Heat-Enriched Fluids”, or SHEF as the Panel has chosen to refer to them, are probably too dense to contribute to instability, spire morphology does suggest the existence in the past of buoyant fluids (Sec. V.B.4).
The influence of these fluids is extremely important both from a scientific point of view and for a practical understanding of how alterations in this input might affect lake productivity. Future Crater Lake research teams should be encouraged to examine further the paleolimnological evidence, particularly with an eye to more accurate dating and higher temporal resolution. Because the sediment deposition rate is so low, resolution is limited, however.
B. The extent of water quality degradation
The current 10-year program at Crater Lake was initiated in part because of concern over a suspected decrease in lake clarity since 1937. Although this concern is legitimate in any case since Crater Lake’s extreme clarity merits protection, the actual evidence for decreased clarity is rather weak.
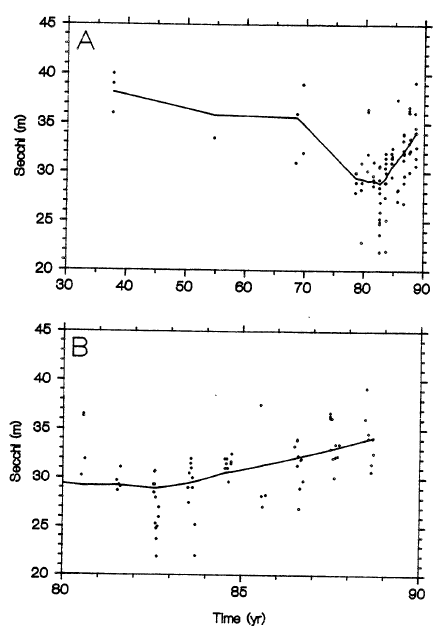
From 1931 through 1942, the water level of Crater Lake was unusually low, 3 to 4 m below current levels, suggesting a time of unusually low precipitation (Redmond 1990). This period also may have been one of reduced vertical mixing, particularly if the probability of storms was reduced at times of minimum lake stability in early spring and late fall. A natural consequence would have been reduced upwelling of regenerated nutrients, a lower annual primary productivity, and probably a lower maximum phytoplankton biomass (Goldman et al. 1989b). This could account for the record high Secchi depths measured in 1937, the first year of Secchi data (Larson et al. 1990). Secchi depths were not measured again until 1954 and then again in 1968 (Larson 1990); in both of these later years, they fell well within the range of the past decade (e.g. 1980, 1987). The 1969 values had a higher maximum than all subsequent years, but it should be noted that a major El Niho/Southern Oscillation occurred in 1969 and could have resulted in extreme lake conditions. At Castle Lake, California, for example, 1969 was a year of unusually low productivity (Goldman et al. 1989b).
Thus, the high Secchi measurements prior to 1970 could have been due to a bias that entered because of the small number of years sampled and the high interannual variability (Fig. 1A). The small number of measurements made within a year combined with high seasonal variability could also introduce bias. As pointed out by G. Larson (1990), the dependence of Secchi depth is extremely sensitive to particle density in clear lakes. The consequences of interannual and seasonal variability for water clarity are therefore most pronounced for low-fertility, ultraoligotrophic waters such as Crater Lake.
Although a statistical test (Dahm et al. 1990, Table 2) seems to imply that water clarity decreased after 1969, the chance bias described above suggests that such a test be considered only as weak evidence. Furthermore, the t-test used by Dahm et al. (1990) is inappropriate if there is actually serial correlation (such as trend) in any of the populations being measured. Alternative distribution-free tests are available for trend detection in the presence of serial dependence and other problems. In addition, from 1982 (when the number of measurements within a single year increased substantially) through 1987, the trend — if one can be substantiated at all — may be one of increasing water clarity (Fig. 1B). The evidence for increased chlorophyll a concentrations (D. Larson et al. 1990, Table 4) or primary productivity (Dahm et al. 1990, Table 3) is as uncompelling as that for decreased Secchi depths.
Unfortunately, the combination of interannual variability and a sparsely sampled — but large — seasonal variability may preclude any dependable conclusions regarding trend for some time. Because of the difficulty of getting higher-resolution data and a time series of measurements of sufficient length, we recommend that more emphasis should be given to the sediment evidence (see above).
C. Nutrients and decrease in water clarity
Several threats to the lake’s clarity can be hypothesized. Ironically, one of these is the input of hydrothermal waters, at least according to the evidence put forward by Dymond and Collier (1990; Section A above). D. Larson et al. (1990) postulate a role for sewage contamination.
Because the conclusions of Collier, Dymond and McManus (1990) are so heavily dependent on geochemical arguments, it is instructive to compare important geochemical fluxes from hydrothermal processes with those from other sources. According to D. Larson et al. (1990, p. 207), nitrate is the limiting nutrient for phytoplankton in Crater Lake. They estimate about 62 x 106 liters of sewage each summer flows into septic tanks on the south rim, with subsequent infiltration into the lake. For a summer season of 90 days this is an average sewage-related flux of about 8 liters/second. For comparison, the calculations based on chemical mass balances in the Dymond and Collier report (1990, p. 111), “yield a total flow of hydrothermal fluid of approximately 290 liters/second”. Thus, the hypothetical SHEF flux is one to two orders of magnitude greater than the estimated sewage-related flux.
However, the nitrate contents of the waters of Lloa’s pool, Palisades pool, and the deep lake are reported as being essentially identical (Collier, Dymond and McManus, 1990, Table 7, p. 108), so the SHEF pools could not be an important source of nitrogen for the very dilute lake water. On the other hand, a spring which discharges at 2 liters/second to the lake, and which Larson et al. (1990) believe to be contaminated by sewage effluent, has a nitrate content enriched by two orders of magnitude relative to that of the lake water. Thus it seems possible that the flux of the most-likely limiting nutrient (N) into the lake may be dominated by anthropogenic sources rather than by hydrothermal inputs. This is particularly evident when we remember that in addition to wastewater from sewage, these anthropogenic sources of nitrogen also include the probability of atmospheric contaminants.
Enhanced algal growth typically occurs as one approaches nitrogen-tophosphorus ratios of from 10-15 to one. Since Crater Lake owes its great transparency to low nutrient content, any increase in nitrogen, the major limiting factor, is certain to enhance algal growth and reduce transparency. As hypothesized for Tahoe and supported by lake-moored dry and wet fallout collectors in Tahoe, increased atmospheric deposition of nitrate could also increase eutrophication of the Crater Lake system. In the case of Crater Lake, though, the atmospheric deposition probably would not be derived from local sources of air pollution, but rather from long-range transport from populated areas where NOx vehicle emissions are common, or from agricultural areas where fertilizer NH3 is heavily used. In order to distinguish among these and other possible alternatives, several pieces of evidence would be valuable:
1. An annual nitrogen budget still needs to be established. From the documents made available to the Panel, it does not appear to be available. Mass balance studies have investigated the major ions, but the absence of nitrate — and not phosphate — from euphotic waters implicates nitrogen as the element most limiting for maximum algal biomass and, therefore, minimum Secchi depth. Nitrogen tends to be limiting in oligotrophic western lakes and, in fact, there appears to be about as much nitrogen as phosphorus limitation in lakes in general (Elser et al. 1990). Particularly important is the atmospheric deposition of N03. Deposition is typically heterogeneous and an appropriate spatial array of samplers is usually necessary. At Tahoe, for example, the difference between shore and midlake stations is marked. Dry fallout as well as precipitation should also be measured. Although dry fallout at Tahoe is only about 10-15% of wet deposition for both N03 and NH4, the percentage can be much higher for other substances, up to 100% in the case of soluble reactive P and 70% in the case of Na (Byron et al. 1989).
2. Synoptic samples are required to document the spatial heterogeneity of Secchi depth, primary productivity and nitrogen ion levels. In the vicinity of the Rim Village, such studies could assess directly the possible impact of the septic system. The annual amount of fixed nitrogen in Rim Village wastewater (2500 kg) is approaching the same order of magnitude as that in precipitation (5000-9000 kg; D. Larson et al. 1990).
3. Compartment model activities should be extended to an analysis of long-term nitrogen dynamics, with a resolution of 1 year. Such modeling activities may be able to differentiate among nitrogen sources that have different time courses. Annual primary production estimates are necessary for such modeling activity.
4. Higher resolution of mixing activity as recommended in the previous peer review is still needed. Following the evolution of the nitrate vertical profile in spring still seems to be a viable possibility for accurately tracing mixing depth, although it would require higher-frequency measurements at the start of each sampling year. Nevertheless, some more accurate measure of annual mixing activity is essential if interannual variability in water clarity is to be understood.
IV. Microbiology of Benthic Mat Communities
A. Introduction and general comments
During the 1989 Panel review meeting we were presented with the findings of widely-dispersed microbial communities that exist in localized areas on the bottom of Crater Lake. These observations were judged to be quite exciting by the Panel, both for the reason that they may be indicators of the SHEF input, and because they may have intrinsic interest as unusual microbial communities. Because of these general and specific interests, several areas of research were suggested to the PI’s:
1) Carbon isotope (S13C) analysis of sediments in the mat areas vs those elsewhere
2) Taxonomic analyses of the mat materials
3) Culture and physiological analyses of the suspected organisms
4) Flux measurements in mat areas of likely critical nutrients
5) Pore water chemistry in mat vs non-mat areas
The PI’s were unable to respond directly to the requests for microbiological data that the Panel recommended after the first review. We wish to stress that Dymond and Collier are not microbiologists, and were dependent on the work of volunteer collaborators. Unfortunately, the collaborator presented a report which included virtually no data, only statements as to what did and did not work. This is not the fault of the PI’s, but, unfortunately, the issues of what the critical biological communities in the mats are, or how fast they may be growing, remain unresolved. Both of these issues are central to assessing the possible importance of the mats to the ecology of the lake and their possible significance in terms of estimating SHEF inputs more enriched in metals than the lake water.
On a more positive note, it is obvious that some substantial progress was made in the identification of new areas of mat formation. This demonstrates that the mats are much more diverse and widely distributed than was previously known. The Panel is in agreement that the funding agencies would have been well-advised to have committed more effort, resources, time and funding to investigating more fully the microbial mats in Crater Lake.
B. Report
1. Experimental design
The major approaches used for mat analyses to this date have been descriptive. Descriptions include gross morphology and distribution of mats, microscopy and electron microscopy of bacterial communities, and bulk chemical analyses of mat samples. While these descriptive results have definite limitations, as a first approach, they have yielded valuable data. As mentioned above, the previous review panel had some specific recommendations that required a different experimental design in addition to description. The experimental design followed to answer these questions was, by and large, not adequate. For example, 1) the methods for culturing and identifying organisms were not documented, 2) a strategy for studying rates in the lab or field was not outlined, and 3) no strategy for isotope fractionation analysis was outlined or attempted.
2. Methods
The methods used for the descriptive part of the program done by the Dymond/Collier group included photography, microscopy, electron microscopy, and bulk chemistry. These methods seem adequate and well described, between the draft report and the published data.
The methods for the more detailed microbiological work were not well documented in this report. It was not possible to tell what was done, nor is it, in general, possible to tell what did or did not work.
3. Results
Descriptive work: The report begins with a recapitulation of the data from 1988. This shows the photography of mat zones from the north wall, some SEM analyses of these mat areas, and a discussion of what they might be, based on morphology of the cultures. This essentially repeats the material contained in the report from 1.5 years ago. This remains a very interesting discovery; whether or not the input to the lake is hydrothermal, or from some other source, the existence of these major communities is an important finding.
Defining the environmental settings of mat communities: One new development involved the identification of further areas of mat growth, and confirmation of what had been seen earlier.
a) One mat area that had been dispersed during sampling in 1988 had grown back by 1989. Unfortunately, no detailed information was provided about these observations. What is meant by dispersed? How large was the mat community? How much organic carbon was involved in the process? If this latter amount were known, it might be possible to get new insights on the fluxes of iron into the lake, or through the sediments.
b) Temperatures as high as 10 0C were observed in 1989, and were primarily along the Chaski Slide area. In probing experiments, temperatures as high as 18.9 0C were recorded, with several values >15 0C. In general these mats (found at approximately 400 m depth) were not as large or well developed as those seen at the north wall site. It is suggested that the venting of fluids is heterogeneous because of the localized distribution of the mat communities. These mats have high concentrations of arsenic and rare earth elements. c) The saline pool mats or communities represented the third group of mat types found in the study area. These communities formed a vast array of morphologies, ranging from well-developed mats associated with small pools to thin coatings or crusts on adjacent rocks and sediments. Some were many meters across, while others were narrow rims around the fringe of the pools. They also tended to be the lowest temperature features (about 6 00). d) In addition to the mat types discussed above, all in the study area from 1988, a major community was found across the lake in the Palisades Point area. This particularly interesting community was a zone 20-30 meters long by 5 m wide, with many small rivulet-like structures. The micro “rivers” that seemed to feed the mats often originated under rocks in the area, and “flowed” into pools that supported mat growth. Temperatures here were on the order of 8.1 OC.
Microbiological results: In a previous paper, Dymond et al. (1989) proposed on the basis of electron microscopic work that these organisms were iron chemolithoautotrophs. The ensuing results were aimed at answering some specific questions about the purported iron oxidizers. All of the data relevant to these questions, and to the specific recommendations of the previous Panel are contained in Appendix E, supplied by Dr. D. Karl of the University of Hawaii. A review of these results is presented below.
C. Pure culture studies
Enrichment cultures were set up on a variety of different media for Sphaerotilus, Leotothrix, Gallionella ferruginea, Thiobacillus thiooxidans (obligate sulfur autotroph), and T. intermedius (facultative S oxidizer). This section was troubling to the Panel, as there are no descriptions of the media used, or the conditions used for growth (T, atmosphere, medium components, etc.).
It is stated that positive enrichments “were common” on all media except those for T. ferroxidans and T. oxidans, both of which are usually thought of as obligate S (and/or Fe) oxidizing autotrophs. No cultures were isolated as pure colonies, nor studied with regard to any of their properties.
D. Enzyme assays
Bacterial mat assemblages were examined for two different C02-fixing enzyme activities, RuBisCo (Ribulose 1,5-bisphosphate carboxylase) and PEP Case (Phosphoenol pyruvate carboxylase). No success was obtained with the assay of either enzyme. Also, no data are shown for any assays, positive controls, other organisms, etc.
E. Dissolved substrate analyses
This project, to look at pore water samples for dissolved organic matter, was discussed, but no data were presented.
F. Other relevant data
Since no pure cultures were obtained, it was impossible to address many of the issues we had suggested in our 1989 review. There is no further information regarding the identification of the mat community, its physiology, growth rates, response to temperature, nutrients, etc.
No attempts were made (or mentioned) regarding the 13C fractionation of mat material, which would have been another way of implicating an autotrophic community.
No attempt was made to look at rates of growth or metal oxidation activity in the lab or the field.
G. Analyses, interpretations, and conclusions
The analyses of the microbiological data are few, since the results are primarily descriptive. There is no substantiation of the identity of these organisms, their physiology, or the flux of material through them.
The refined studies of the bacterial communities still need to be done to support the statements that are made in the report. There is no indication, other than structural, that the community is composed of the organisms suggested, or that they are actually metal oxidizing bacteria growing autotrophically. There is no information, other than anecdotal, regarding their growth rates, their metabolism, or their relation to metal oxidation in the lake. This latter point may be critical. If the fluid fluxes are on the order of 200 liters/sec, and if iron is in the range of 0.5-1.0 mM in the pore waters, and if the lake has been in steady state for the past 100 years, then in the upper layers of the bottom sediments, there should be on the order of 5-50 kg of iron as iron oxide per square meter of sediment. If this amount is not there, then there may be some fundamental role that the mats are playing that could be used to the advantage of understanding the environment. However to do this, the dynamics of the mat community must first be understood.
Third and finally, the mat communities described here may be unusual and unique, or they may be simply larger analogues of well known systems in other lakes that are driven by anaerobic groundwater input. Because of the lack of bacteriological results it is not possible to distinguish between these two alternatives.
H. Additional studies and suggestions
for the future Stable isotopes: If future work is to be done, the establishment of the nutritional base of the mat communities via carbon isotope studies should definitely be included as a high priority.
Identification and characterization of the mat communities: Determining whether or not the Crater Lake system is unique will be greatly aided by identifying the members of the community. It will be straightforward to compare it to other iron-driven systems, and to look for differences and similarities. While it seems likely that the system is a unique one, there is not yet a sufficiently good data base to defend this conclusion.
Field and laboratory studies of bacterial physiology: There are a wide variety of questions regarding fluxes of nutrients, iron, fluids, etc., which could be well addressed using pure cultures in the laboratory, and later, careful analogous studies in the field. With regard to the question of establishing an iron budget, it will be critical to know the role of the iron bacterial communities in maintaining this budget or quantifying fluxes in the lake.
V. Geophysics and Geology
A. Introduction and general comments
The investigators have focused their attention on geochemical techniques, and particularly on geochemical techniques with which they are familiar. Their interest in, and apparent understanding of, the local and regional geological, geophysical, and even continental, geothermal setting of the lake, is limited. Thus, some important factors are lacking for the evaluation of the significance of their results. This still remains a shortcoming which could be considered serious from the point of view of the National Park Service objectives.
B. Report
1. Experimental design
The experimental design had only limited emphasis on geology and geophysics and the geochemical investigations were focused on a small area of the lake bottom based on the findings of Williams and Von Herzen (1983). The Von Herzen study had some limitations in terms of very limited penetration into the sediments that cause uncertainties with the results. Thus, some further evaluation of the historical data was suggested by the Panel report of July 1989. Because of the limited use of geological/geophysical techniques to investigate the hydrologic system, the report could be described as incomplete.
The Panel felt that an oversight in the report was the lack of an attempt to put the data and models presented for Crater Lake into a regional context which compares them with the broad range of volcanic and hydrothermal phenomena displayed in the Cascade Ranges. Because of the very extensive and quite recent volcanism of Mt. Mazama, it would not be surprising to find abundant and pervasive hydrothermal phenomena in the area. The eruptions of Merriam Cone and Wizard Island, which occurred as recently as 4,000 yr. B.P., suggest that the magma chamber beneath the volcano was not completely depleted by the catastrophic eruption which formed the crater about 6850 yr. B.P. (Bacon and Lanphere, 1990). In fact, some Panel members were surprised by the small-scale and limited-range of the SHEF phenomena which have so far been described by the Crater Lake studies.
However, because of high rainfall, permeable rocks, and steep topography, surface hydrothermal manifestations in the Cascades are usually highly modified or often suppressed. On the other hand, useful comparisons to Crater Lake could be made, for example, with the situation at Newberry Crater, where geothermal exploration has revealed the existence of a large high-temperature system. Such a comparison might help focus the discussion of why significant hydrothermal features at Crater Lake were so difficult to find.
2. Methods
The methods of hydrothermal geology/geophysics used were primarily visual investigation from the submersible of the lake bottom, focusing on the detailed study area (pp. 19-31, draft report). Even allowing for a limited role of these studies in the overall research plan, there are some major problems. As an example, the submersible passes right by a fault scarp on the bottom (tape sequence, Plate 4) but the location, the orientation or the displacement was not recorded. Such faults and fracture zones are the most likely localizers of fluid leakage to the lake floor and hence their locations are extremely important to establish or document. The existence of the area of study at the end of a major regional normal fault zone (and significance of such a location) appears to be unknown to the investigators.
No physical property measurements of any sort (such as porosity or permeability measurements) have been made on core samples.
The investigators have also ignored the information and studies of Cascade volcano geothermal systems, so the report has no context for the reviewers or the NPS to evaluate the results and implications.
3. Results
The results can best be understood in the context of the hydrologic systems associated with stratovolcanoes. The researchers have oversimplified the hydrologic systems expected to two end members rather than the full spectrum of groundwater thermal and chemical possibilities. Such systems consist of a shallow high flow rate groundwater system (flow rates of meters to 100’s of meters per year, extremely low TDS, short resident times) that dominates the surface manifestations in an area of high rainfall such as the Cascades. Examples of this type of groundwater system include nearly all of the springs in the Crater Lake region. This system gives way at depths of 200 to 500 m to a regime of much lower flow rate and more complete and complicated interaction with the rocks and other deep waters. The diversity of flow rates (m/year or less) and chemistry is great. Most Cascade volcanoes have summit fumaroles with temperatures of over 90 OC. For example, Mount Hood has a fumarole at its top with a temperature of 92 OC and an estimated thermal energy discharge rate of 25 MW. Acid alteration zones are often found over the top of such fumarole zones if there is a perched water table. In the core of such a system, high temperature geothermal systems can be found, Such systems are usually NaCl solutions of neutral pH.
4. Interpretations and conclusions
One of the primary conclusions of the study is that there are inputs of hydrothermal fluids into the bottom of Crater Lake. The estimated composition of the fluid is shown in Table 7 of the Draft Report. The Panel suggests that this fluid at its zone of discharge into Crater Lake be referred to as a “salinity- and heat-enriched fluid” (SHEF). On the basis of the present data, null hypothesis 4a rather than 5 (Dymond, Panel presentation, 1/14/91) is the best way to describe the findings, i.e., there is injection of a SHEF into Crater Lake at a rate of 300 +100 liters/sec with the majority of this injection occurring in the southeast basin. This fluid contains the gases carbon dioxide and helium, which can be readily detected in the overlying lake water. There is a large 3He excess component in this water and the C02 is old (see Sec. VI). We note that there may be inputs and outputs to and from Crater Lake from the groundwater system of compositions not inventoried that could impact the calculations of input flow rates and chemistry and the interpretations drawn therefrom. The data allow the comparison of the inferred fluid to waters of various sorts in the Cascades (Table 7, Collier et al. 1990). It is interesting to note that these SHEF waters are so dilute that they could still be classified as potable with respect to the major chemical elements they contain. The water chemistry as it is presently known is not characteristic of medium or high temperature geothermal systems in the Cascade Range (see Mariner et al. [1990] for analyses of hot spring waters in the Cascade Range). The water is, therefore, less characteristic of high temperature geothermal systems as we presently know them. Some of the surface springs in the vicinity of Mt. Mazama are more concentrated than these SHEF inputs. For example, an unnamed calcium bicarbonate spring on Minnehaha Creek has a TDS of about 640 mg/liter, and Soda Spring on Minnehaha Creek has a TDS of 3035 mg/liter. The analyses of Llao’s and Palisades pools (SHEF) differ markedly for the Mt. Mazama springs listed in Thompson et al. (1990, Table 1A), in having Mg > Ca >> K, a situation which is most unusual for typical geothermal waters (Ellis and Mahon 1977), which are normally depleted in magnesium and enriched in K.
Although the outflow temperature is about 19 OC, the fluids, when circulating at a slow rate, may have lost temperature to surrounding rock. In the Galapagos, Dr. Gieskes recalls “hydrothermal fluids” of 25 0C, but silica thermometry which indicated an original temperature of about 350 0C. The temperature is typical of a depth of circulation of 300 to 500 m below the bottom of the zone of rapid cold groundwater flow described above. For example, Swanberg and Morgan (1979) found that the average silica temperatures of water from wells in the western United States are 55 to 95 0C, equal to or higher than the fluid inferred to charge Crater Lake.
C. The discovery of spires near Skell Head
The discovery of high (10 m) spires near the base of the caldera walls is important for several reasons. First, the spires are predominantly amorphous silica (Fig. 50a) according to the draft report. This suggests precipitation from fluids at higher temperatures than any yet measured in the present study. The height of the spires supports vigorous outflow and/or very buoyant, high-temperature, fluids.
Although it is not clear that the system that formed the spires is still active, their discovery is perhaps the best direct evidence yet that “hydrothermal” processes have operated through the floor of the lake. Furthermore the morphology of these spires suggests that the fluids from which they precipitated were rising buoyantly. This is not the case with the SHEF waters, as can be seen by comparing Plate 15 and Plate 16. Similarly in Figures 42 and 45 of the draft report, it is apparent that the SHEF water is dense enough to flow down slope. Thus it seems unlikely that it could be contributing in a major way to density instability which increases mixing in the lake.
These SHEF bottom flows appear to be vigorous enough to form localized erosional channels, but the fragile and complex structure of the bacterial mats suggests that the exit velocity of the venting SHEF water is not great enough to destroy or modify them.
In the oral presentation, Dr. Dymond commented that the spire sample contained bands of material. From microprobe studies, these bands are apparently accompanied by variations in the composition of the spire. In particular, iron and silica appear to vary inversely (Fig. 49). These results also support hydrothermal precipitation in a varying chemical environment. Dr. Dymond also commented that the banding in the sample appeared to form an incomplete cross section through rings of precipitated material. Here, some photographs in the report would have been helpful to the reviewers.
The spires are also important because they appear at the base of the caldera wall and, like the Palisades Point features, they exist far from the Chaski slide region. In the original Panel recommendations, we urged the researchers to look for features outside the Detailed Study Area. While they did not plan a major search for thermal features, it was fortunate that the dives located some. These later discoveries indicate that some of the features could not possibly be associated with groundwater flowing down the Chaski Slide. In addition, the discovery of the spires is consistent with the hypothesis that thermal waters are flowing up along the caldera walls or ring fractures.
In the report, the authors suggest that the spire features are rather young (<50 years) because they found no spire debris or broken spires. In addition, the spires have only a light dusting of sediment, even in their crevices. These observations are consistent with the age estimate. However, in the video shown for the Panel, the spires appeared more broken up, which suggested to one Panel member a greater age. In addition, the survey of the spires was necessarily brief and perhaps evidence of debris could be found on further research. While the details of the spire sample recovery are not clear, it appears that only half a chimney was recovered from a top of the spire. If so, this suggests that the missing part of the spire was broken off sometime in the past. It is regrettable that more time was not spent on investigating the spires by the submersible since the petrological data on these interesting features could be valuable. It may eventually be possible to date them by a study of their actinide chemistry, and to determine their temperature of formation using oxygen isotopes.
In any event, the Panel suggests that although estimates of spire age are premature at this time, the spires are extremely interesting and potentially important. Future work on the known spires and a search for additional ones could certainly be interesting and potentially rewarding.
D. Comments on mixing calculations
1. Experimental design
The researchers have made a serious effort to understand the mixing dynamics of the lake. This is a scientifically important problem, and is definitely important in understanding the role of the SHEF inputs at the bottom of the lake. The bulk of this material was presented orally to the Panel and, unfortunately, was not included in the draft report. The Panel feels strongly that the material presented to the Panel that was not included in the draft report should definitely be included in the final report.
In the opinion of the Panel, these mixing models should be included in the final report as the required sublake influxes of He, Rn, S04 and Cl, etc. constitute perhaps the best indirect evidence of hydrothermal processes. Enhanced concentrations of such dissolved components are characteristic of warm springs in volcanic terrains. For example, mixed bicarbonate/sulfate waters are often the product of reaction by steam-heated groundwater with volcanic rocks, and the source of sulfate is often oxidation of H2S, concentrated into and transported by the steam phase.
On the other hand, it does not necessarily require that the hydrothermal system is active today. As Nathenson and Thompson (1990) point out, we could have a low flux of high salinity water or a high flux of low salinity water, to give the same mass flow of the necessary components. Sufficient inflow of relatively dilute groundwater, such as that sampled from Llao’s Pool, which had traversed and leached rocks which were altered in previous hydrothermal episodes, would achieve the same results. On the other hand, Collier and Dymond’s modeling reaches conclusions somewhat similar to those of Nathenson and Thompson (1990); however, discussion of the similarities and differences between the modeling by the OSU and the USGS teams would be a valuable addition and should be included in the final report.
2. Results
Seasonal mixing is well documented in the data. A series of nitrate profiles strongly suggests the accumulation of nitrate in the bottom waters of the lake. This vertical mixing is not as complete at the bottom of the lake, as revealed by the seasonal temperature profiles and the presence of higher nitrate levels near the bottom (Larson et al. 1990). Sampling in February and March would improve our understanding of the mixing dynamics of the lake.
The SHEF are relatively dense, and pools on the bottom appear to form. Further, there is evidence from the 3He and the ion profiles for mixing. There is also evidence of spill-over (horizontal flow) of south basin bottom waters into the northeast basin and some vertical mixing near the bottom is apparently occurring there. Further, elevated ionic concentrations at midwater levels (<300 m) support the hypothesis that bottom waters (with an SHEF component) are incorporated into the lake as a whole.
3. Comments on the conclusions
The Panel, with the available data, was unable to reach a consensus as to whether or not the input of SHEF fluids at the bottom of the lake is sufficient to strongly influence the mixing of Crater Lake. Since lake mixing is so dominated by seasonal variations and climatic changes, the role of SHEF fluids in the mixing patterns is not yet known. One note of caution should be mentioned. The accuracy of chemical analyses has improved markedly during the last 80 years, and this is particularly true for elements like Na and Li.
E. Comments on “box” modeling effort
1. Experimental design
The design of models includes the assumptions used, in this case the steady-state assumption, and the nature of model inputs. One of the goals of the modeling was to test the hypothesis that ionic concentrations in the lake today are a result of the original volcanic inputs four-to-seven thousand years ago.
Model inputs are derived from several hydrological studies. While values for various parameters may be disputed, the authors attempted to be conservative in their use of values throughout their study. For the modeling process, the authors used a finite difference approach to examine the time-history of chloride and lithium levels in the lake. The finite difference approach is standard and noncontroversial. A reference for the code used would, however, be helpful for the reviewers.
2. Results and conclusions of the “box” model
The major result of this section is that the lake water has a relatively short memory with respect to its entire history. Thus, ionic concentrations soon after initial formation are not reflected in the present-day chemistry of the lake. This conclusion is not in question and the issue of steady-state assumptions is not critical. That is, they argue that the original volcanic eruptions cannot be the source of present chloride concentrations. Whether or not more recent, but possibly extinct, fumarolic inputs have played a role is not known.
In their presentation to the Panel, the authors extended this box model. They “reversed” the model by forcing it to be non-steady state and found that the concentration of sodium would have had to change over the last 80 years if it resulted from an impulse input sometime in the recent past. This change would have been seen within the range of previous observations. Since it hasn’t, they conclude that sodium inputs have been steady state during the last few decades. This result also appears sound.
3. General comments about the “box” model
The box model approach is useful because it indicates that present-day ionic concentrations must be maintained by a steady-state input. Alternatively, current ionic concentrations could be maintained by frequent, small, transient inputs. If this is actually the case, then these inputs have occurred recently and perhaps are occurring now.
Obviously, a box model cannot locate sources, but the mass balance argument does indicate that SHEF do substantially contribute to the lake’s salt balance (see Geochemistry Sec. F and the Summary). One question that remains is whether or not SHEF also have a major impact on the thermal structure of the lake and on its mixing characteristics. This question is addressed to the best of the Panel’s ability in the Limnology section.
VI. Geochemistry
A. Introduction
Since the last review (Goldman et al. 1989a) of the Dymond/Collier preliminary report, several suggestions have been followed up, which included more detailed sampling of fluids in bacterial mats as well as of pore fluids in the sediments, both in areas of background sediments as well as in areas of bacterial mats. In addition samples have been obtained from the newly (1989) discovered “saline” pools. No work has been done as yet on oxygen isotope systematics and we strongly urge that this be carried out in the future. It is essential that all samples are preserved in glass containers for the oxygen isotope analysis.
The Panel noted that, on page 18, the report lists at least thirteen other investigators who received water and sediment samples from Dr. Collier and Dr. Dymond as part of their sampling of Crater Lake. The report presents very few results from these other related geochemical studies. Apparently, those studies were regarded as being of lower priority and presumably they were not funded by this project. Many on the Panel would have preferred giving a higher priority to this work than some of the work actually reported; for example, a major deficiency remains the lack of data on light stable isotopes.
B. Inorganic constituents
The previous work on the chemical composition of the lake waters (Na, Cl, K, Mg, Ca, Mn, H4SiO4) has continued, but, more importantly, it has been extended to include analyses of bacterial mat fluids, pool waters, and interstitial waters.
The concentration-depth profiles of Figure 21 are useful, but it would be more effective to show on an enlarged plot all of the data, including those of previous years and of the North Basin. This would allow comparison of small differences and features that get lost in composite plots with error bars (which can be indicated separately in these graphs). The main features, like those in the salinity-depth profiles, will remain and will demonstrate the relative importance of South Basin with respect to salt inputs into the lake.
The ion-ion correlations (especially if consistent labeling is used) can now be extended to include the data of mats and pools. The importance of such plots is well demonstrated in Figure 22 (Na vs Cl), but Figures 23 and 24 could be improved by including all water data now available. In addition other ion-ion plots should be made in a similar manner. The inclusion of Ca from GC1 and GC8, but the omission of the other pore water data, which appear to fall along the main regression line, is awkward. It appears, if anything, that Ca in GC1 and GC8 were different from the majority of the cores. This, of course, begs the question of how Ca behaved in cores GC2 and GC3 in the North Basins. Did they also show little increase in Na but relatively large increases in Ca (and Mg)? Indeed, why was Mg of the pore waters omitted from the correlation plots in Figure 23?
As such the ion-ion correlations suggest strongly that the higher salinity fluids in the South Basin have a common compositional origin. This is also evident from 3He ion correlations which follow later in the report.
C. Pore water chemistry
In accordance with the Panel’s previous suggestion, detailed pore water studies have been carried out. The data presented orally by Dr. C.G. Wheat were more extensive than included in the report and we urge the reporting of the entire data set in Appendix F, including data on Cores GC2 and GC3. This is of importance as is the inclusion of the data in the ion-ion correlations, as shown by Dr. Wheat during his presentation to the Panel.
It would be helpful for future readers if the potential error of the calculated fluxes is indicated. There is no doubt that the box cores were taken closer to the mats than were the gravity cores. It would be particularly helpful to reviewers if a short description of the location of the box cores vis-a-vis the bacterial mats were provided in the report.
D. Oxygen mass balance
The question arose in our discussions as to the importance of dissolved reduced iron with respect to oxygen depletion in the deep waters (Figure 19; Appendix A). There is a clear correlation between dissolved oxygen and Na as well as with temperature (Figure 19). Considerations of dissolved nitrate distributions should be made in order to determine how much decomposition of organic material would contribute to the oxygen utilization. In addition the question arises if this calculated oxygen utilization can to some extent be the result of mixing processes between lake waters and anoxic input waters, or whether oxidation of reduced iron in the bacterial mats leads to a significant chemical oxygen demand. In other words, with the information available on dissolved iron in pore fluids and mat fluids, can a reduced iron flux be estimated that would yield an estimate of the “chemical” oxygen demand. Indeed, such an estimate might well be of considerable importance with respect to the problem of the genesis of the iron rich bacterial mats.
E. Rare Earth elements
The data on REE compositions of lake waters, pool waters, as well as pore waters presented by Dr. Gary Klinkhammer are most relevant and they should be included in the final report. The additional data provided to the Panel on Mazama solids and aluminosilicate debris from the caldera wall indicated no Europium (Eu) anomalies in the solid phases, but the dissolved REE data of Dr. Klinkhammer indicated a pronounced positive Eu anomaly. Such anomalies have also been observed in fluids emanating from hydrothermal vents on oceanic ridges (Campbell et al. 1988).
This information is of great significance and deserves further elaboration in the report with regards the potential origin of this Eu anomaly. Does it require interaction with the rocks at higher temperatures?
F. Geochemical modeling
The presentation of the geochemical models in the preliminary report (pages 107-115) was felt to be much less clear than that presented orally by Dr. Collier to the Panel. We urge a revision to make this section more conformant with the oral presentation. This is particularly important because the calculations help emphasize that the ion flux deficits are very large when the bottom input is ignored in the geochemical mass balance. A sensitivity analysis of this mass balance is appropriate. In any case, the “hydrothermal” flux constitutes well over 50% of the total input flux. The results of the mass balance calculations constitute one of the important conclusions of the work: Inputs of thermally enhanced (-19 0C) and more saline fluids (475-750 mg dm-3) into the bottom waters of the South Basin are the principal contributors to the salt balance of the entire lake.
G. Geothermometry
The analyses of Llao’s and Palisades pools (SHEF) differ markedly from the Mt. Mazama springs listed in Thompson et al. (1990, Table 1A), in having Mg > Ca >> K, a situation which is most unusual for geothermal waters (Ellis and Mahon 1977), which are normally depleted in magnesium, and enriched in K. The Panel recommends caution in using semi-empirical geothermometers which have been derived from data on much more concentrated chloride-dominated solutions at higher temperatures (Fournier 1981) on the cold dilute fluids, with the unusual chemistry, shown in Table 7, page 108. For example, experience shows that the most reliable geothermometer for geothermal waters which have mixed with cold dilute water is the Na K Ca geothermometer with a Mg correction (Fournier 1981). The authors claim to have used this Na K Ca thermometer, with a Mg correction factor of R = 100 Mg/(Mg+Ca+K), for the SHEF waters of Table 7. For the Palisades Pool water this gives a correction factor R of more than 100, showing just how meaningless this widely used geothermometer is for compositions such as these. Collier and Dymond, on page 123 of their report, cite the temperature of 165 OC for Llao’s pool based on the Na Li thermometer. In normal hydrothermal fluids ratios of Na/Li are controlled by reactions between water and minerals such as albite and mica, and so this thermometer is most appropriate for moderate to high temperature systems. Geothermal waters have Na/Li ratios in the range of 20 to 5,000 (Ellis and Mahon 1977, Table 2.3). However the concentration of Li in the SHEF waters is very low, with ratios of Na/Li of 106, showing an inappropriate use of geothermometry.
H. Geochemistry of sediments
Much of the sediment chemistry discussed in the present report results from earlier studies by Collier and Dymond (1988, 1989, 1990). This information is summarized in Figure 46 and indicates the results of the three component analysis of the sediment composition. One of the shortcomings of the section is the lack of mineralogical and petrographic data on the sediments and precipitates. See also our previous comments on the silica spires.
The chemical information has been extended to an analysis of selected samples of Fe-crusts, Si-crusts, spires, and pool sediments (Table 5; Figures 47, 48, and 49). On page 98 it is argued that pool sediments are enriched in sulfur and carbon and reference is made to Table 5, which is erroneous. If Table 5 is examined, one notes no data for C and S. This reference in the draft report needs corrective action.
The observations on the spires is interesting – see also comments in Section V – perhaps some work on oxygen isotopes of the amorphous silica might be useful. However, it would be of great importance if the age of the spires could be established in an unambiguous manner. If these spires are only 60 years old, it would imply that episodic inputs of higher temperature fluids must occur in Crater Lake.
The section of REE in the sediments can now be strengthened with the availability of the data on dissolved REE as presented by Dr. Klinkhammer. As this section stands at present it does not contribute as much as it could. New sediment core analyses on box cores BC6 and BC8 needs to be expanded and put together with relevant profiles of dissolved Fe and Mn (if available). As such again this section (page 101) is incomplete and should be strengthened. It is our understanding that more work is planned on these cores and, if anything, this section could be left in abeyance. Mass balance calculations would be relevant to understand the large enrichments in solid iron.
I. Investigations with dissolved environmental isotopes and chlorofluoromethanes (freons)
1. General comments
Measurements of the distributions of a number of dissolved environmental isotopes and freons (CFC-11, CFC-12) have played a central role in advancing understanding of processes in Crater Lake in the present study and in those of a number of previous investigations. These data have been critical in quantifying the rates of vertical mixing of the water column, fluxes of hydrothermal ions and dissolved gases into the deep lake, locus of hydrothermal inflows, accumulation rate of bottom sediments and other processes involving interactions between the atmosphere and the lake surface. These data represent key calibration measurements for model calculations of lake processes and qualitative indicators of several essential components of conceptual models of the dynamics of Crater Lake geochemical and hydrologic budgets.
Because of the central role of these data in a number of considerations, it would be very helpful to have all of the published data for a number of dissolved isotopes and freons collected in the final report in the form of tables and figures. In particular, the parameters for which summary tables should be included are 3He, 4He, 222Rn, 226Ra, 14C, 13C, CFC-11 and CFC-12. The team of scientists involved in the study of hydrothermal processes in Crater Lake have, in general, made effective use of the above group of tracers, within the limitations of time, budgets and availability of collaborators.
2. Helium isotopes
These data lie at the heart of the development of improved understanding of chemical budgets in Crater Lake over the past five years. The approach employed for these tracers was based primarily on the experience gained from extensive research by many scientists in chemical oceanography over the past fifteen years. Since there are only a few laboratories capable of 3He measurements in environmental samples, it was critical to obtain this collaboration. We are generally in agreement with the approach taken in application of He isotope measurements in Crater Lake, but also have suggestions for improving this aspect of the study. We believe the data reported unequivocally demonstrate the dominance of supply from mantle sources of helium isotopes dissolved in the deeper waters of the lake. Since these influxes near the bottom are rapidly lost to the atmosphere by gas exchange at the lake surface, the observed excess of 3He and 4He in the deeper waters can only be maintained by continued influx of mantle-derived He to the lake and what appears to be partial mixing during some winters.
We were especially impressed by the He isotope data obtained for the “end-member” samples collected from the bacterial mats and enhanced salinity pools on the lake floor. These samples could only have been acquired using a submersible, and their measured 3He and 4He concentrations provide key anchors upon which much of the subsequent chemical and hydrothermal budget calculations are based. However, a word of caution here. The influx of 3He into the lake is in itself an interesting phenomenon; however, that its total flux is entirely associated with inflow of hydrothermal fluids remains an assumption which needs to tested by further study, as the permeability of fractured rocks to helium is very high.
We have two recommendations for treatment of He isotope data in the final report. First it would be very helpful to have all of the measured values for Crater Lake compiled together. At present the data can be found only by locating several references in addition to the draft report of October 26, 1990. The total number of separate samples analyzed for He isotopes over the past five years is less than three or four dozen. These could easily be presented in a single table and a limited number of essential figures, most of which are already present in the draft report.
Secondly, we suggest that the contribution of dissolved 3He by decay of tritium should be explicitly included in the discussion. Although this addition would not alter the conclusions as presented in any significant way, it would help communicate the unusual nature of helium isotope budgets in this lake, compared to one of similar depth and tritium concentrations which did not have a source of mantle helium to the deep waters.
3. Radon and radium
Considering all of the tracers measured, the activities of 222Rn in vertical profiles from the lake provide the most sensitive indicator of the primary locus of groundwater influx. The depth interval at which the-dominant input occurs to the deep lake can be unequivocally established from the 222Rn data because of the extremely high ratio of this tracer in groundwaters, including SHEF fluids, to 222Rn concentrations in the open lake (about a factor of 105). Although these data alone are not sufficient to distinguish between inflows of ambient temperature groundwaters and hydrothermal fluids, when combined with the other observations discussed in the report, they help locate quite precisely the depth at which addition of SHEF fluids is occurring. Because of the short half-life of 222Rn (3.825 days), the strong signal at a depth of about 450 meters can only be sustained by the continued influx of SHEF waters.
Measurements of 222Rn in the lake obviously required a major investment of effort in collection and analysis. We suggest that it would be quite valuable to collect all of the 222Rn and 226Ra measurements from Crater Lake and springs within the Park in a single table (the 1989 data could be left in Appendix I as currently tabulated, and also included in a second Rn/Ra table with all of the previous data for these parameters.
4. Dissolved 14C and 13C
Measured values for these parameters were not included in the draft report of October 26, 1990. However, 14C values for 4 samples from Crater Lake and 2 additional samples from a caldera spring and East Lake were obtained by the principal investigators a few days prior to the meeting of our Panel on January 14, 1991, and were discussed in the oral presentation. These data were extremely interesting, and one of the samples from an enhanced salinity pool at the lake bottom was essentially free of any 14C, indicating that it is very old (greater than 25,000 years). This observation of old 14C is consistent with a magmatic origin of the inorganic carbon in the hydrothermal influx near the lake bottom.
Dymond interpreted this observation as being consistent with a magmatic (He said “mantle” in his presentation) origin of inorganic carbon in a hydrothermal influx near the lake bottom, and suggested that it could not be explained by any plausible model of groundwater influx with no magmatic carbon. However, in the opinion of some of the Panel members, alternative models are perhaps as or more plausible than the one preferred by Dymond. The rather limited data available indicates that 14C is below detection limits in the dissolved carbonate and bicarbonate in the SHEF water (which contains only 4.96 – 8.70 mM of total dissolved carbon). Dymond’s inference is that it must be magmatic because, if the reservoir for this carbon was the atmosphere, it would have to be much older than the eruption which formed Crater Lake. However, this does not require that the carbon is derived directly from the degassing of an underlying magma chamber. One possible reservoir for the dead carbon beneath the lake is carbonate veins, which, as is typical for volcanic terrains, are ubiquitous in the vicinity of Mt. Mazama. For example, core samples from a 415 m deep borehole, drilled 5 km SE of Crater Lake, contain abundant calcite veins with associated zeolites and quartz, which formed in multiple stages of authigenic mineralization. U-Th geochronological techniques reveal that this authigenic mineralization was episodic, with varying degrees of oxidation, and it ranged in age from 140 Ka to >350 Ka BP (Hull and Waibel 1989). Because these ages are similar to the K-Ar ages of the silicic volcanic rocks, most of this mineralization was contemporaneous with construction of the Mt. Scott stratovolcano, on the SE flank of Mt. Mazama. Therefore, the carbon in these veins could be “dead” and appear to be “mantle” carbon. This interpretation, however, was not unanimous, since some Panel members believe that the proposed source of dead carbon from carbonate veins could not sustain the necessary flux of 14C-free carbon dioxide over thousands of years, noting that it has never been proven to be a major component of volcanic C02 in any andesitic volcano in the Pacific Northwest or elsewhere. There are a very small number of special volcanic situations with large volumes of associated carbonate rocks (e.g., East Africa) where it would be plausible to consider such a model, but Crater Lake is not one of these.
As for the rocks of Crater Lake, Bacon and Lanphere (1990, p 26) point out that, except between Pumice Point and Wineglass, the rocks at lake level are “everywhere subtly to severely hydrothermally altered”. Most likely such alteration would form in the crater-fill, immediately after the climactic eruption and again during the Merriam Cone and Wizard Island eruptions. Subsequently cold, oxidizing meteoric water (lake water?) would encounter warmer, previously hydrothermally-altered, rocks beneath the lake floor, and dissolution of hydrothermal carbonates and sulfides would give rise to the mildly alkaline bicarbonate/sulfate SHEF waters.
These 14C data were measured by accelerator mass spectrometry in Zurich, through collaboration with Peter Schlosser (formerly at the University of Heidelberg) and his colleagues. Unfortunately, 13C data obtained by accelerator mass spectrometry are subject to poorly-defined fractionation processes which prevent them from being of use here. We strongly recommend that the 14C data be included in the final report, accompanied by a detailed discussion of their important implications in this study.
5. Dissolved freons
Vertical profiles of these tracers in Crater Lake for August 1989 are given in a figure and brief discussion in Appendix G of the draft report. These data provide the most sensitive indicator of the time-scale of ventilation of deep waters in the lake and provide the basis for estimation of a mean renewal time of 2 years for these waters. The freon data are so critical to model calculations for a number of parameters, including helium isotopes, and dissolved oxygen, that we suggest discussion of this data be expanded and provided earlier in the report. At the minimum, details of renewal time calculations based on freons should be provided, perhaps in summary table form in Appendix G, and referred to earlier and with more emphasis in the final report.
6. Suggestions for future applications of environmental tracers in Crater Lake
Research at Crater Lake over the past three-four decades has revealed a great deal about the details of its chemical, physical and biological processes. These findings indicate that Crater Lake provides almost a unique location for obtaining accurate estimates of atmospheric input of tracers such as fission products and tritium, integrated over a number of years, as well as receiving an influx of the warmer, enhanced-salinity fluids (SHEF) near the bottom which play a critical role in the chemical budgets of the lake. With improved understanding of lake processes, the site has become even more valuable as a location for study of long-term processes involving atmosphere surface water exchange, environmental controls of phytoplankton communities in highly oligotrophic lakes and their effects on water clarity, dynamics of microbial communities based on the influx of anoxic waters, as well as a number of other limnological subjects.
From the results obtained in this study and others, there appears to be considerable potential for further exploitation of tracer measurements in Crater Lake, especially involving He isotopes, carbon isotopes and freons. One issue of potential conflict with other areas of research involves 14C. The distribution of this tracer in the lake is extremely sensitive to the input of magmatic carbon or carbon isotopes to the deep lake. To preserve the value of this natural tracer, it is essential that great care be taken in future measurements of primary production by incubation with radiotracer levels of 14C to prevent contamination of the lake, or of any samples to be used for natural 14C levels. This kind of potential conflict illustrates the value of some form of sustained planning and oversight of long-term research involving Crater Lake by scientists with a broad range of backgrounds.
J. Additional studies and suggestions for further work
1. Light stable isotopes
Among an otherwise comprehensive array of geochemical studies described and interpreted in the Draft Report on Crater Lake, there is one omission which the Panel regards as being particularly unfortunate; i.e., no investigations of light stable isotopes by the OSU team are reported. This is puzzling as among the co-investigators to whom water and sediment samples were sent, the Principal Investigators mention Dr. Alan Mix, of Oregon State University, who received splits for oxygen and hydrogen isotopic analyses. However, the report presents no stable isotope data either from that study or from the studies of other investigators.
The Panel views light stable isotopes as being of particular importance in understanding both the limnological and the hydrothermal geochemistry of Crater Lake. The extensive literature developed during the last three decades on measurement and interpretation of isotopic ratios of hydrogen, carbon, oxygen, and to a lesser degree of sulfur, in the study of hydrologic systems in general and of hydrothermal systems in particular, testifies to the importance of this versatile and cost-effective approach (Craig 1961; Ellis and Mahon 1977; Fournier 1981; Gonfiantini 1986; IAEA 1979; Thompson et al. 1990).
The omission of stable isotope chemistry from the draft report of Collier, Dymond and McManus is therefore unfortunate. We suggest that a much higher priority should have been given to stable isotopes, and an appropriate level of funding should have been assigned to acquiring and interpreting the necessary data, particularly with respect to samples of the salinity- and heat-enriched fluid inputs to the floor of the lake. This deficiency should be remedied in the future, even if it is necessary to arrange for the work to be carried out by competent isotope geochemists outside of OSU. There are numerous investigators in the U.S.A. with the necessary analytical facilities and experience in using these techniques on hydrothermal rock/water systems of many kinds.
The lack of stable isotopic data in the draft report is also puzzling in that its authors are aware of the utility and importance of such an approach. For example, on page 116 of their report, they cite the work of colleagues in the USGS (Thompson et al. 1987, 1990) which shows that hydrogen and oxygen isotopic ratios of waters from Crater lake and surrounding springs indicate that its waters “fall off the meteoric water line and follow a reasonable evaporation trend”. However, the USGS studies (Thompson et al. 1987, 1990) use stable isotope ratios to address much more than this single issue. Thompson and his co-workers compare the hydrogen and oxygen isotopic ratios of 26 samples of Crater Lake waters with 28 samples of cold springs discharging from the flanks of Mt. Mazama, and one from Diamond Lake, a lake about 20 km north of and 300 m lower than Crater Lake. Their results show that: (a) as might be expected, the isotopic ratios of the cold-water subaerial springs lie on the meteoric water line; (b) samples of Crater Lake water obtained from throughout the water column show that the lake is isotopically well-mixed; (c) the Crater Lake waters are heavier isotopically than the spring waters due to evaporation from the lake; and (d) a few of the isotopically light spring waters are more chloride-rich than typical local springs by amounts which approach the chloride concentration of the lake.
However, Thompson and his coworkers did not have samples obtained during the submersible dives. It would have been highly desirable to compare their published data with the hydrogen and oxygen isotopic ratios of water samples from lake bottom water, from the pore waters in the lake-bottom sediment, and from the “anomalous”, slightly-warmer and more-saline, pools on the lake bed. On page 109 of their report Collier, Dymond and McManus infer, from the calculated Na/Li geothermometer temperature of 164 OC, that the waters in these small pools of SHEF have been cooled from a moderate-temperature hydrothermal source. If this were the case, their oxygen isotopes would exhibit characteristic ratios, due to exchange with the rocks in the hot zone, which are quite different from the evaporation trends observed by Thompson et al. (1987, 1990).
If, as might be expected, it were to be found that these different waters have characteristic hydrogen and oxygen isotopic signatures, we might then develop mixing models which, in turn, could be used to estimate endmember compositions of the different components of the hydrologic system, and specifically of the fluid which gives rise to the SHEF pools and bacterial mats on the lake floor.
If suitable water samples (stored in sealed glass containers) were available now, or were to be collected in the future, analysis of isotopic ratios of carbon and of sulfur might also be attempted, although this would require larger samples due to the lower concentration of these elements. Certainly, hydrothermal components in this system should have distinctive isotopic ratios, depending on their sources, equilibration temperatures, and water/rock ratios.
Studies of light stable isotopes should be extended to encompass geothermometry and rock/water ratios, by adding data on appropriate solids. Fresh and altered dacites and andesites, lake bottom sediments, bacterial mats, and the silica spires are obvious targets. Successful acquisition of such data would allow more precise estimates concerning the nature and temperature of any hydrologic system which may underlie Crater Lake. Such isotopic ratios could and should be used to address the following issues:
(a) The relationship of the local precipitation from different storm patterns and of cold-water subaerial springs to the world meteoric water line.
(b) Comparison of these data to the isotopic ratios of water samples from different levels in the lake, to lake bottom water, and to the anomalous, warmer SHEF forming the more saline pools, and pore waters which apparently enter the lake through its floor.
(c) If characteristic isotopic signatures are found for these different water sources, we could then develop mixing models which, in turn, could be used to estimate end-member compositions of the different components of the hydrologic system, and specifically of the fluid(s) which gives rise to the enhanced salinity pools and bacterial mats on the lake floor. The next step would then be to carry out mass balance calculations for the inputs and outputs of water and dissolved species of carbon and possibly of sulfur to the whole hydrologic budget of the lake. These calculations would enhance and extend the related mass balance modeling based on the element analyses which are included in the report on p. 107-117. If the data permit, it would also be possible to use these isotopic data to provide limits on estimates of geothermometry and possibly of water/rock ratios.
(d) A secondary but important aim of such isotopic studies would be to extend the geothermometry and rock/water ratios, by adding data on appropriate solids, fresh and altered dacites and andesites, lake bottom sediments, and bacterial mats. The silica spires are also obvious targets. If appropriate data on such materials is added to that on the fluids, we should be able to make more precise calculations concerning the nature and temperature of the hydrothermal system which has been postulated to underlie Crater Lake.
VII. Summary
A. Important results from the 1989 and 1990 field seasons The Panel is impressed by the effort made by Collier, Dymond and their coworkers to determine if hydrothermal inputs are present in Crater Lake. The data has been primarily descriptive and geochemical in nature, reflecting the expertise of the researchers and limitations of submersible and ROV research. Their work supports the following conclusions:
1. The Panel agrees that a slightly warmer and more saline water (SHEF) is entering the lake bottom.
2. The SHEF strongly influence the major element geochemistry and environmental isotope compositions of the lake waters.
3. The SHEF fluid “venting” is associated with interesting features. The bacterial mats are particularly unusual and fascinating. Descriptive evidence indicates that these mats are dynamic features. The presence of pools of relatively high density enhanced salinity water on the lake bottom is also an interesting observation. Descriptive evidence indicates that these pools form from the sinking and transport of fluids from vents or seep zones, some of which find their immediate source under rocks.
4. The work described in the 1990 report helps establish that SHEF includes a “magmatic” component, and that the He isotope composition is dominated by a mantle or magmatic source leaking into the lake.
5. The Panel notes the discovery of water with temperature in excess of 15 OC, which is appreciably higher than regional meteoric water, that is entering the lake near the bottom. It should also be noted that a reasonable geotherm within a Holocene volcanic edifice could reach these temperatures at a depth of 0.5 km.
6. The discovery of the Palisades Point features is important because it demonstrates the SHEF are not restricted to the Chaski Slide portion of the Detailed Study Area. However, water column measurements indicate that the greatest influx of fluids occurs in the South Basin, which tends to support the decision to utilize most of the submersible vehicle observation time within that basin.
7. The discovery of the siliceous spires at Skell Head indicates that influx of higher temperature buoyant fluids has occurred on the lake bottom some time in the past. The time when this influx occurred cannot be established from observations available up to the present.
8. There is evidence that the SHEF fluids are mixed in the bottom layer and that advection into the East Basin occurs at the depth of the sill between the South and East Basins.
9. The freon data reported provide the most sensitive indicators currently available of the time scale of deep water ventilation and establish the deep water renewal time to be about two years, assuming a steady-state vertical mixing process. This finding is a critical new result which helps constrain the magnitude of chemical fluxes from SHEF fluids into the deep waters of the lake, averaged over the mean vertical mixing time.
10. The 222Rn activities observed in samples of deep water clearly establish the depth and general location at which the SHEF fluids are delivered to the deep waters of the lake. The distribution of this tracer in the lake water provides unequivocal evidence of influx of high 222Rn fluids to the deep waters of the lake at the time of sampling during August 1989.
B. Other comments
While the above results and conclusions are both interesting and important there remain some uncertainties about the origin and characteristics of the SHEF inputs to the deep waters of Crater Lake. These uncertainties include:
1. The role of the SHEF fluids in the mixing of Crater Lake cannot be defined at the present time. Mixing dynamics are not well established from the available data, and improved sampling is recommended as part of any future monitoring program. Nitrate levels suggest that the lake is not completely mixed to its deepest level.
2. The nature of the system that supplies SHEF fluids to the lake bottom is very poorly defined. The size of the reservoir and the maximum temperatures in the reservoir are not constrained by the present data. The possibility that Crater Lake is underlain by a large high-temperature system still remains to be proved or refuted. Oxygen isotope work would be very helpful, as well as more reliable heat flow data, in resolving this issue. One Panel member believes that the work reported has established that the SHEF fluids form from reactions of lake or spring water with hydrothermally-altered volcanic rocks. This alteration may have occurred during earlier volcanic episodes. He argues that the SHEF water chemistry is not consistent with being derived from a moderate or high-temperature hydrothermal system.
3. Arguments that the siliceous spires at Skell Head (which strongly indicate high temperature fluid input) are “recent” features are not conclusively supported by existing evidence. The submersible did not make a thorough study of the features, and the video tape shows the features to appear broken up. Without better knowledge of water movement, the lack of sediments is not conclusive evidence of recent activity.
4. The nature of the bacterial mats is still unknown despite recommendations from the previous Panel. The Panel recognized the investigators’ efforts to secure volunteer help in this area; still, very little quantitative information about mat growth or metabolic rates has been obtained.
5. A geological model of the hydrothermal system cannot be made. The hypothesis that SHEF fluids enter the lake along the ring fractures that bound the caldera remains largely untested. We appreciate the limitations of submersible or ROV observations in collecting structural information. Even obtaining the strike of features is very difficult. However, until such data is obtained, the geological context of the SHEF fluids remains unknown.
VIII. References
Bacon, C.R. and M.A. Lanphere. 1990. The geologic setting of Crater Lake, Oregon, p. 19-27. In E.T. Drake, G.L. Larson, J.Dymond and R. Collier, (eds.), Crater Lake: An Ecosystem Study. Amer. Assoc. Advancement Sci., Pacific Div.
Bates, R.L. and J.A. Jackson 1980. Editors, Glossary of Geology, Second Edition. American Geological Institute, Falls Church, VA. 751 p.
Byron, E.R., C.R. Goldman, and S.H. Hackley. 1989. Lake Tahoe Interagency Monitoring Program: Ninth Annual Report, Water Year 1988. Tahoe Research Group, Inst. of Ecology, Univ. California, Davis, CA. 78 p.
Campbell, A.C., M.R. Palmer, G.P. Klinkhammer, T.S. Bowers, J.M. Edmond, J.R. Lawrence, J.F. Casey, G. Thompson, S. Humphris, P. Rona, and J.A. Karson. 1988. Chemistry of hot springs on the Mid-Atlantic Ridge. Nature 335:514-519.
Collier, R.W., and J. Dymond. 1988. Studies of hydrothermal processes in Crater Lake. A preliminary report of field studies conducted in 1987 for the Crater Lake National Park. Oregon State Univ., College of Oceanography Ref. #88-5. 49 p.
Collier, R.W., and J. Dymond. 1989. Studies of hydrothermal processes in Crater Lake. A report of field studies conducted in 1988 for the National Park Service. Oregon State Univ., College of Oceanography Ref. #89-2. 79 p.
Collier, R.W., J. Dymond, and J. McManus. 1990. Studies of hydrothermal processes in Crater Lake, OR: a report of field studies conducted in 1989 for the National Park Service. Draft. Oregon State Univ., Corvallis, OR.
Craig, H. 1961. Isotopic variations in meteoric waters, Science 133: 1702-1703.
Dahm, C.N., D.W. Larson, N. S. Geiger, and L.K. Herrera. 1990. Secchi disk, photometry, and phytoplankton data from Crater Lake: long-term trends and relationships, p. 143-152. In Crater Lake: An Ecosystem Study. E.T. Drake, G.L. Larson, J. Dymond, and R. Collier (eds). Pacific Div., Am. Assoc. Adv. Sci., San Francisco, CA.
Drake, E.T., G.L. Larson, J. Dymond, and R. Collier. 1990. Crater Lake: An Ecosystem Study. Pacific Div. American Assoc. Adv. Sci., San Francisco. 221 p.
Dymond, J., and R.W. Collier. 1990. The chemistry of Crater Lake sediments: definition of sources and implications for hydrothermal activity, p. 41-60. In Crater Lake: An Ecosystem Study. E.T. Drake, G.L. Larson, J. Dymond, and R. Collier (eds). Pacific Div., Am. Assoc. Adv. Sci., San Francisco, CA.
Dymond, J., R.W. Collier, and M.E. Watwood. 1989. Bacterial mats from Crater Lake, Oregon and their relationship to possible deep-lake hydrothermal venting. Nature 342:673-675.
Ellis, A.J. and W.A.J. Mahon. 1977. Chemistry and Geothermal Systems. Academic Press, New York. 392 p.
Elser, J.J., E. Marzolf, and C.R. Goldman. 1990. Phosphorus and nitrogen limitation of phytoplankton growth in the freshwaters of North America: a
review and critique of experimental enrichments. Can. J. Fish. Aquat. Sci. 47:1468-1477.
Fournier, R.O. 1981. Application of water chemistry to geothermal exploration and reservoir engineering, p. 109-144. In L. Rybach and L.J.P. Muffler (eds.), Geothermal Systems: Principles and Case Histories. John Wiley & Sons, New York, 359 p.
Goldman, C.R. 1990. Summary of Crater Lake studies and comparison with the early stages of eutrophication of Lake Tahoe, p. 213-221. In E.T. Drake, G.L. Larson, J. Dymond, and R. Collier (eds.), Crater Lake: An Ecosystem Study. AAAS, Pacific Div.
Goldman, C.R. and A.D. Jassby. 1990. Spring mixing depth as a determinant of annual primary production in lakes, p. 125-132. In M.M. Tilzer and C. Serruya (eds.), Large Lakes: Ecological Structure and Function. Springer-Verlag, New York.
Goldman, C.R., et al. 1989a. Crater Lake: Peer review of research program and recommendations for additional investigations of possible hydrothermal activity. Report to the National Park Service, Seattle. 14 p.
Goldman, C.R., A. Jassby, and T. Powell. 1989b. Interannual fluctuations in primary production: meteorological forcing at two subalpine lakes. Limnol. Oceanogr. 34:308-321.
Gonfiantini, R. 1986. Environmental isotopes in lake studies, p. 113-168. In P. Fritz and J. Ch. Fontes (eds.), Handbook of Environmental Isotope Geochemistry, Vol. 2. Elsevier Scientific Publ. Co. Amsterdam.
Herdendorf, C.E. 1982. Large Lakes of the World. J. Great Lakes Res. 8(3):379-412.
Hull, C.D. and Waibel, A.F. 1989. U-Th disequilibrium dating of authigenic calcites in the Mazama (MZI-IIA) geothermal well. Oregon. Geothermal Resources Council Transactions 13:157-163.
International Atomic Energy Agency (IAEA). 1979. Isotopes in lake studies. Proceedings of an advisory group meeting on the application of nuclear techniques to the study of lake dynamics. International Atomic Energy Agency, Vienna, 29 Aug.-2 Sept. 1977. 285 p.
Larson, G. 1990. Status of the ten-year limnological study of Crater Lake, National Park, p. 7-18. In Crater Lake: An Ecosystem Study. E.T. Drake, G.L. Larson, J. Dymond, and R. Collier (eds.). Pacific Div., Am. Sci., San Francisco, CA.
Larson, G., et al. 1990. Crater Lake Limnological Studies: 1989 annual report. Oregon State University, Corvallis, OR.
Larson, D.W., C.N. Dahm, and N. S. Geiger. 1990. Limnological response of Crater Lake to possible long-term sewage influx, p. 197-212. In Crater Lake: An Ecosystem Study. E.T. Drake, G.L. Larson, J. Dymond, and R. Collier (eds.). Pacific Div., Am. Assoc. Adv. Sci., San Francisco, CA.
Mariner, R.H., T.S. Presser, W.C. Evans, and M.K.W. Pringle. 1990. Discharge rates of heat and fluid by thermal springs of the Cascade Range, Washington, Oregon, and northern California. J. Geophys. Res. 95:19,517-519,531.
Nathenson, M. and J. M. Thompson. 1990. Chemistry of Crater Lake Oregon, and nearby springs in relation to weathering, p. 115-126. In E.T. Drake, G.L. Larson, J. Dymond and R. Collier (eds.), Crater Lake, an Ecosystem Study. Amer. Assoc. Advancement Sci., Pacific Div.
Paerl, H.W., R.C. Richards, R.L. Leonard, and C.R. Goldman. 1975. Seasonal nitrate cycling as evidence for complete vertical mixing in Lake Tahoe, California-Nevada. Limnol. Oceanogr. 20(1):1-8.
Redmond, K.T. 1990. Crater Lake climate and lake level variability, p. 127-142. In Crater Lake: An Ecosystem Study. E.T. Drake, G.L. Larson, J. Dymond, and R. Collier (eds.). Pacific Div., Am. Assoc. Adv. Sci., San Francisco, CA.
Smith, R.C., J.E. Tyler, and C.R. Goldman. 1973. Optical properties and color of Lake Tahoe and Crater Lake. Limnol. Oceanogr. 18(2):176-188.
Swanberg, C.A., and P. Morgan. 1979. The linear relation between temperatures based on the silica content of groundwater and regional heat flow: A new heat flow map of the United States. Pure and Applied Geophysics 117:227-241.
Thompson, J. M., L.D. White, and M. Nathenson. 1987. Chemical analyses of waters from Crater Lake, Oregon, and nearby springs. U.S. Geol. Surv. Open-file Report 87-587. 26 p.
Thompson, J.M., M. Nathenson, and L.D. Whit, 1990. Chemical and isotopic composition of waters from Crater Lake, Oregon, and nearby vicinity, p. 91-102. In E.T. Drake, G.L. Larson, J.Dymond and R. Collier (eds.), Crater Lake: An Ecosystem Study. Amer. Assoc. Advancement Sci., Pacific Div.
Utterback, C.L., L.D. Phifer, and J.R. Robinson. 1942. Some chemical, planktonic and optical characteristics of Crater Lake. Ecology 23(1):97-183.
Williams, D.L. and R.P. Von Herzen. 1983. On the terrestrial heat flow and physical limnology of Crater Lake, Oregon. J. Geophys. Res. 88(B2):1094-1104.
Other pages in this section
*** previous title *** --- *** next title ***