Composition of the Water of Crater Lake, Oregon by Walton Van Winkle and N. M. Finkbiner 1913
Van Winkle, Walton and N. M. Finkbiner (1913). “Composition of the Water of Crater Lake, Oregon.” The Journal of Industrial and Engineering Chemistry
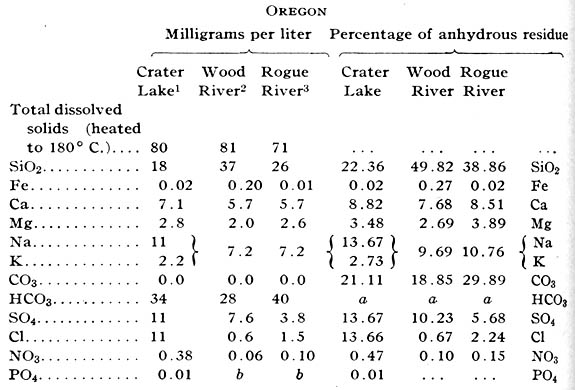
Crater Lake, in the heart of the Cascade Mountains of Oregon, is one of the most interesting spots in the United States, both to scientist and to tourist. Popular accounts of its beauties have been published in magazines and its chief features of scientific interest have been admirably set forth.’ But though analyses of typical rocks are given in Diller and Patton’s paper no analysis of the water of the lake has yet appeared in print, and the subjoined analysis may, therefore be useful to those interested in this remarkable body of water.
The following brief description of the lake, based or Diller’s report and on personal observations by the senior author, makes the analyses more readily understandable. Crater Lake is situated in a geologically recent caldera occupying the site of a once lofty volcanic peak – Mount Mazama – in the midst of the Cascade Range, about 55 miles northeast of Medford Oregon. The rim of the caldera is from 7,000 to 8,000 feet above the sea, and the lake surface was 6,175 feet above sea level in 1908. The inner slope of the rim bears, in some places, a sparse growth of pine, but in many others its walls drop sheer to the water’s edge, here and there fringed by steep talus slopes. The surficial area of the lake is approximately 21 square miles, and its drainage basin is only about 6 square miles larger. The greatest depth of water is 1,996 feet, and a cinder cone projects more than 760 feet above the surface at the western extremity of the lake to form Wizard Island. Precipitation is more than 70 inches a year, occurring chiefly as snow in winter. Evaporation is less than 55 inches, and this, with loss by percolation, almost completely balances the inflow, there being no surface outlet to the lake. Some of the water may find its way by percolation into Rogue River, but more of it probably goes southeastward appearing as springs in the drainage basin of Klamath Lake.
1 J. S. Diller and H. B. Patton, “The Geology and Petrography of Crater Lake National Park,” Prof. Paper U. S. Geol. Survey, 3 (1902); J. S. Diller, “Geological History of Crater Lake,” Department of the Interior, 1912.
As loss by percolation is only about one-third of the loss by evaporation, analyses of the water of the lake may be expected to give chemical evidence of slow concentration. That the analysis really shows concentration almost identical with that of other surface waters of the region is explained, however, by the fact that no sedimentary materials are exposed, the andesites, dacites, and basalts forming the basin of the lake, I being nearly insoluble in the cold water, and, therefore, incapable of rapidly increasing its content of mineral matter. Concentration of chlorides is great as compared with that of other materials, an indication of the concentrated character of the water. As the published analyses of rocks indicate that almost no chloride exists in these formations, it is possible that the high percentage of that radicle in the water is due almost entirely to accumulated “cyclic” chlorine precipitated with the rain and snow. The unexpectedly high percentage of sulfates is possibly caused by solution of sulfur that remained in the bottom of the caldera in a more or less oxidized condition at the cessation of active volcanism. No other features of the analysis seem unusual, when it is compared with the accompanying analysis of waters collected from Wood and Rogue rivers in the same season.
U.S. Geological Survey
Salem, Oregon
Other pages in this section
*** previous title *** --- *** next title ***